Are current practices of dissolution testing just to meet the regulatory requirements?
It appears so.
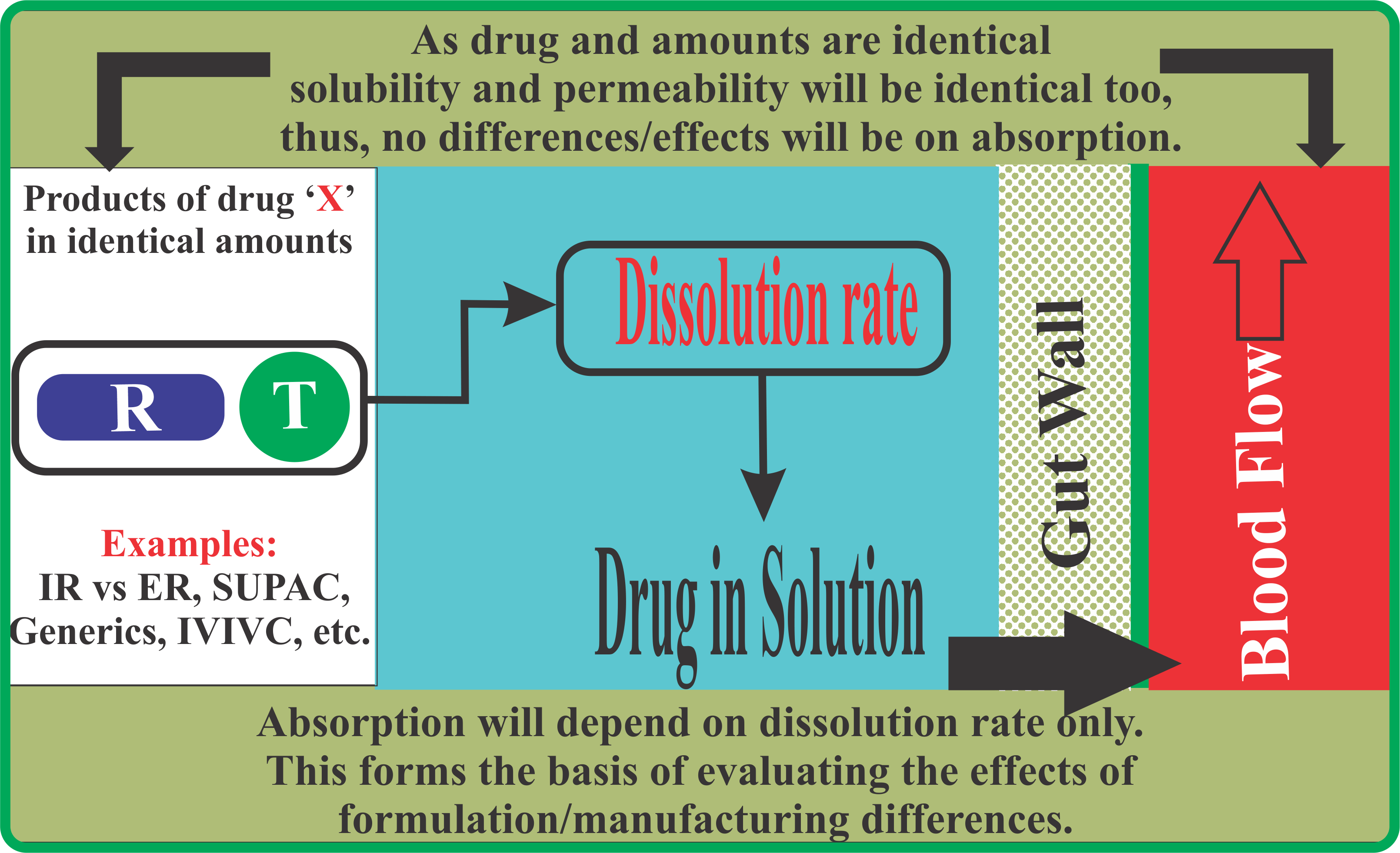
In principle a dissolution test is conducted to assess the quality of a drug product based on its in vitro drug release (dissolution) characteristics. However, in recent years it has clearly been shown, based on experimental as well as computer simulation studies, that the tests, in particular using paddle and basket apparatuses, are not capable of providing relevant and reproducible results. Thus, test results do not reflect the true quality of the products.
Another disturbing aspect in this regard is a concern that most of the tests, i.e., experimental conditions, employed are product dependent. With the use of product dependent experimental conditions it is not possible to establish whether the results, thus, the product quality (good or bad) is a reflection of the product or the use of a particular set of experimental conditions. An analyst, at will, can make a product look good or bad (acceptable/unacceptable) or a product of either slow or fast release type by adjusting the experimental conditions appropriately.
Therefore, current practices of dissolution testing appear to be practices aimed at meeting the regulatory requirements rather than assessing the quality of drug products.
It has further been shown that if the artifacts of the paddle and basket apparatuses are adequately addressed, where apparatuses are able to provide efficient product-medium interaction, a dissolution test can be made a useful analytical tool as intended. For a further detailed discussion on this topic please see the publication.
A bio-relevant dissolution test?
Bio-relevant dissolution tests are highly desirable and are often referred to. It appears that such tests are commonly understood as intuitive expectations rather than a clearly defined scientific objective. This creates confusion, misunderstandings and leads to lack of progress in this regard. To avoid such confusion, the following may be considered as an appropriate definition:
“A bio-relevant dissolution test is a test which is conducted using experimental conditions representing GI tract environment, in particular intestinal, and capable of predicting in vivo response comparable to the actual blood concentration-time profiles obtained from the bioavailability/bioequivalence studies.”
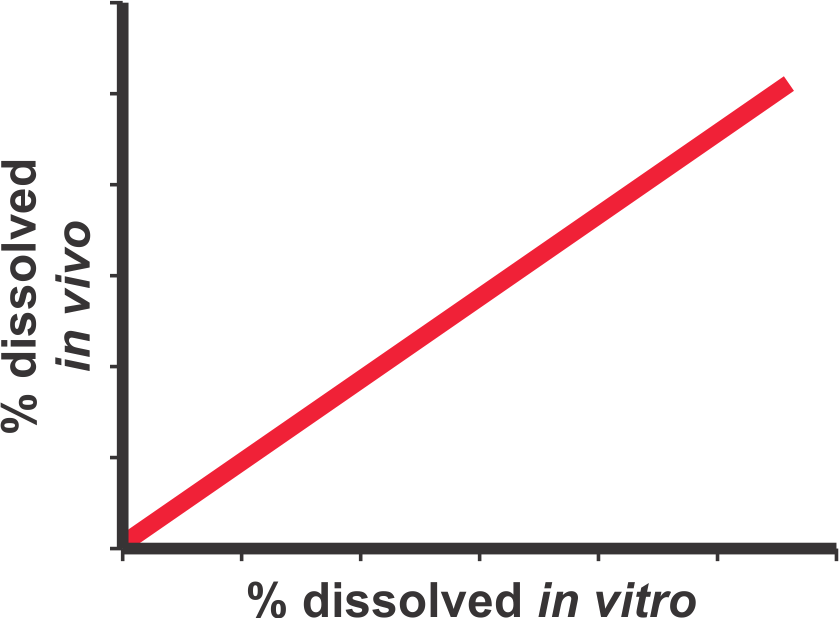
It is essential to understand that a bio-relevant test must be conducted using appropriate and bio-relevant experimental conditions. Use of non-physiological test conditions such as de-aeration of dissolution medium, product dependent experimental conditions, inefficient or lack of stirring/mixing within dissolution vessels, etc., are to be avoided. Furthermore, the test conducted with appropriate test conditions must also be able to predict in vivo (blood) drug concentration-time profiles. For example, the predicted profiles should at least be able to differentiate between fast (e.g. IR) and slow release (ER) products as these would be from an in vivo study. Such a test should then be used to evaluate potential drug release characteristics of a test product. For further discussion of the subject and detailed methodology to calculate drug concentration-time profiles from dissolution results please see the links (1, 2).
Different IVIV Relationship Terminologies
-
IVIVC: (In Vitro-In Vivo Correlation).
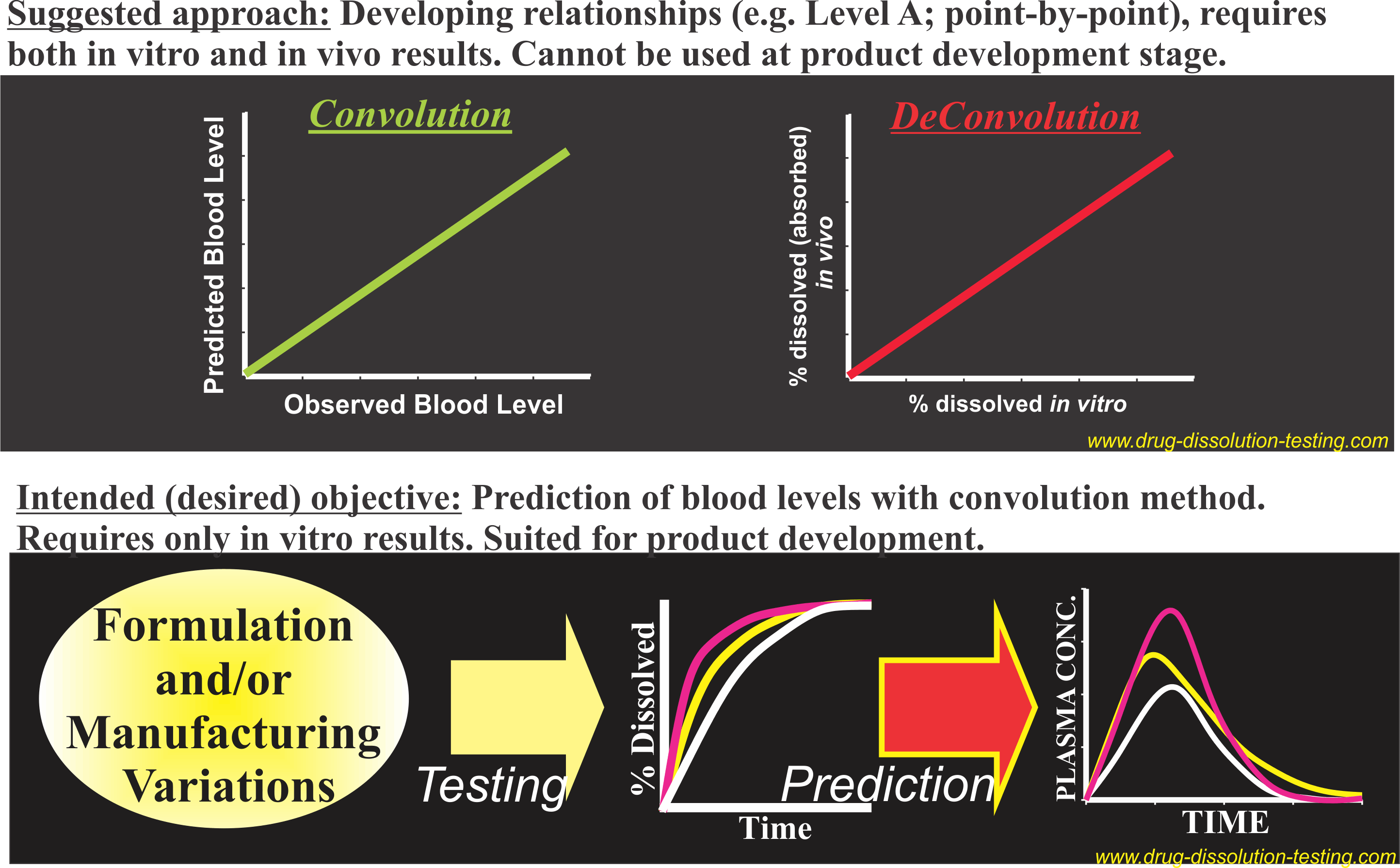
This is the most commonly referred terminology and appears to originate from US FDA guidances. It define the IVIVC as, “A predictive mathematical model describing a relationship between an in vitro property of a dosage form (usually the rate or extent of drug dissolution or release) and a relevant in vivo response, e.g., plasma drug concentration or amount of drug absorbed”. Common interpretation: Usually a point-to-point relationship is to be established between the in-vivo and in-vitro parameters of the same time points (e.g., in-vivo fraction drug absorbed vs in-vitro fraction drug dissolved) by applying a mathematical technique of deconvolution. Practical limitations: Both in vitro and in vivo data are required for the same product. The procedure does not provide predictability but reflects relationship between in vitro (dissolution) and in vivo (fraction absorbed/dissolved), if successful. As results are compared/related using a single product, the approach may not be used for the comparison of products, for their formulation/manufacturing attributes. Continue reading
Simplification to a “universal” dissolution test
It is often considered to be highly unlikely, if not impossible, to have a universal dissolution test. Let us explore whether this is a myth or reality?
Most commonly used dissolution methods are those described in the USP monographs (500+) and FDA database (500+). Examined closely, one observes that the majority (~76%) of the tests employ a paddle apparatus at 50 rpm (see US FDA database). Evaluation of USP monographs most likely would also lead to the same conclusion.
As for the dissolution medium, majority (~60%) of the tests employs water or aqueous based buffers in the pH range of 5 to 7.
If one considers the two previous practices together, it appears that we already have a “sort of” universal test which is paddle at 50 rpm using medium having pH in the range 5 to 7, which may be water or a buffer. Furthermore, it should also be noted that these conditions are commonly applied for both IR and ER products. Then why do not we use these “universal” test conditions, and have so many (hundreds) sets of dissolution experimental conditions?
One of the most common deficiencies in using this set of experimental conditions is that the results obtained are often slower and highly variable than expected. This is a normal and expected behavior of paddle spindle as the spindle rotation speed of at 50 rpm does not provide efficient product/medium interaction (figure) and movement of solvent (medium) upwards. The issue in this lies with one of the suggestions to address this limitation, which is to increase the rpm. Therefore a significant number (~18%) of tests are conducted at higher rpm of 75-100. However, there are no established criteria available to select an appropriate rpm, other than to obtain the expected dissolution characteristics of the product. Therefore, an analyst has to select a higher spindle rotation speed (rpm) arbitrarily. Interestingly, this practice of selecting the higher rpm is contrary to the popular belief/recommendation that a desirable characteristic of a dissolution test is that it should be discriminating. Continue reading
Dissolution-Absorption Link
Drug Dissolution Testing Mosaic
Dissolution Test For Nicotine Polacrilex Lozenges
In response to a query, I have provided the following opinion on the above mentioned topic. I thought this topic may have general interest, thus posting it on the blog as well. Comments/critiques are always appreciated.
_______________________________________________________________________________________________
Thanks for your interest in my work and the website.
Concerning your question regarding dissolution test for Nicotine Polacrilex Lozenges, first of all I must say that I personally have not worked with such products. Therefore, consider my advice as a theory (quick thoughts) which may be workable with some experimental work.
As you stated that the release of drug (nicotine) from the lozenges occurs in 30 minutes in humans, however, in vitro test requires 8 hours for the release. Obviously somewhere there is a mismatch between in vitro and in vivo environments. Considering that there is a higher pH (6.2 to 7.4) in the oral cavity than in the intestinal tract, the suggested use of a higher pH of 7.4 for dissolution appears appropriate. My preference would be around 6.0
To me, however, there appear to be two issues here. First, there may be a lack of needed stirring and mixing within the dissolution vessel. I am of the view that the use of the basket/paddle apparatus would be the least efficient choice here. Nicotine Polacrilex is an ion-exchange complex, thus may require some modest “shake” to pull the drug out of the polymer. Thus, it may require a different mechanism of stirring, may be a crescent-shape spindle as I have proposed, or something of that nature which should be good and efficient in moving the medium from the surface and in providing mobility to the lozenges.
The second aspect is related to chemistry. The complex between nicotine and Polacrilex is ionic. Thus, you may need some form of competitive cation in the medium, which would facilitate the pull-out or replacement of nicotine from the ion-exchange. Otherwise ion-exchange may keep the nicotine from releasing, which would result in an extra ordinary delay in release (8h?). It is to be noted that the working at pH 7.4 (dissolution) is not very favourable to this dissociation, as the pKa of nicotine is 8.5. Having said that please keep in mind that if you are going to try a competitive cation, this has to be a bio-relevant one, otherwise, your dissolution test will lose bio-relevancy. Perhaps, CTAB may be tried, a commonly suggested solublizer (surfactant) for dissolution testing.
Hope this will help.
Operating principle of a dissolution tester (Paddle/Basket)
The main operating principle of a paddle/basket (or vessel-based) apparatus is to provide a precise and controlled stirring and mixing mechanism at 37 C. In reality, from the operational aspect a beaker with a magnetic stirring bar may be considered equivalent to a dissolution tester if the rpm of the stirrer is precisely controlled and beaker content can be maintained at 37C. Therefore, it is important to note that operation of a dissolution tester should be as simple as any other stirring device.
 To achieve time savings and consistency in results, the current dissolution apparatuses come in units of 6 or more stirrers with appropriate mechanical and electronic controls. However, operating principle remains the same whether the apparatus is based on a single or multiple stirring units.
To achieve time savings and consistency in results, the current dissolution apparatuses come in units of 6 or more stirrers with appropriate mechanical and electronic controls. However, operating principle remains the same whether the apparatus is based on a single or multiple stirring units.
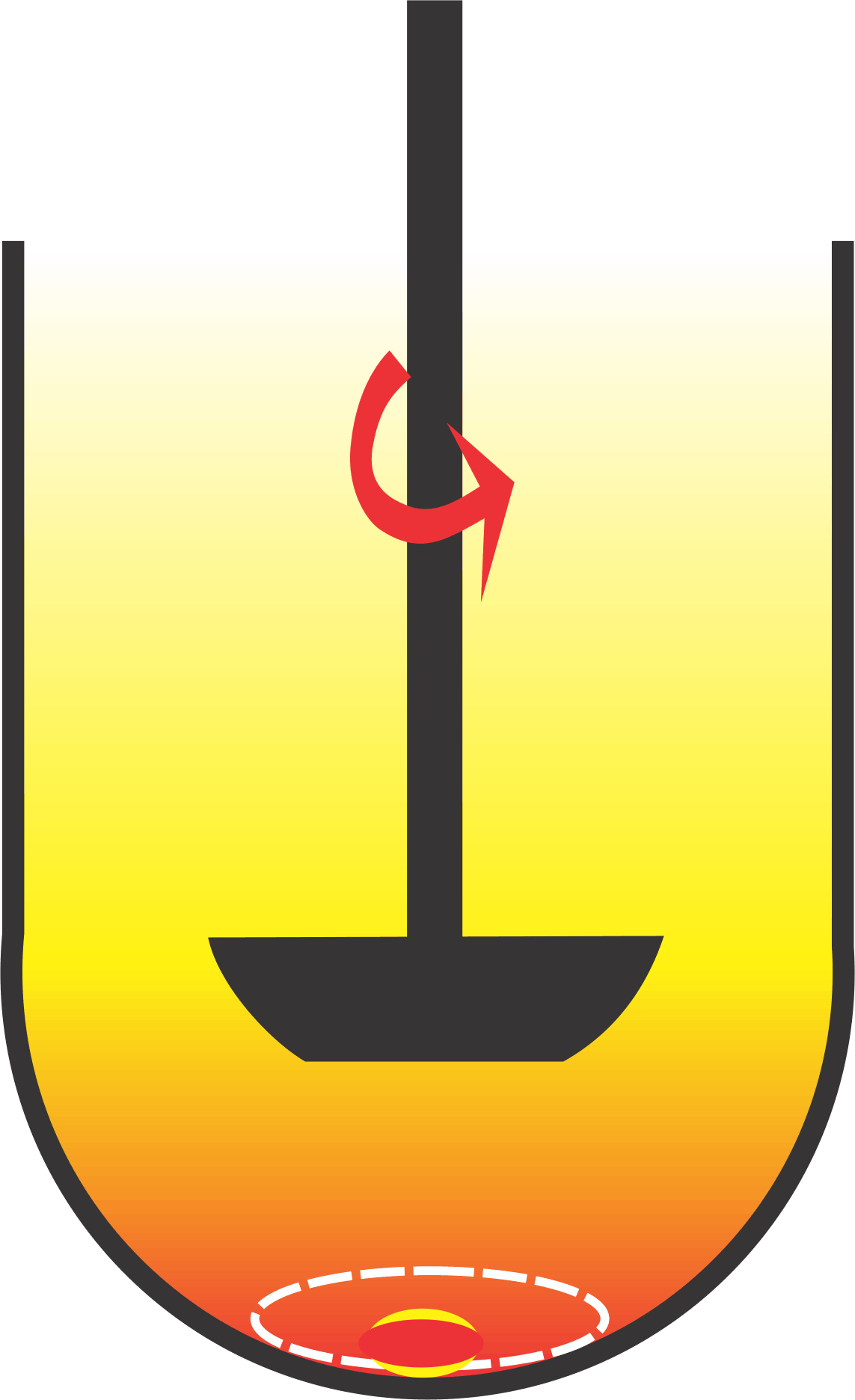
Generally, stirrers (or stirring devices) come with their limitations. For example, a magnetic bar may not be suitable for mixing viscous or solid materials because bars lack sufficient torque and the stirred contents are not easily move able. Similarly, basket/paddle stirrers cannot be used as mixers for solid materials (tablets/capsules) as these settle at the bottom of the vessels where flow of the liquid (medium) is low to negligible. A representation of lack of interaction between solid/liquid with such apparatuses is shown in the figure, which is known as “cone formation”. Therefore, by its nature basket/paddle stirrers (or apparatuses) cannot be used where thorough mixing (interaction) of solid/medium is required, such as what occurs in the GI tract.
Furthermore, the random settling positions of a product (tablet/capsule and their particles) at the bottom of a vessel add variability to this mixing, thus dissolution results would be erratic and unpredictable.
To sum up, the operating principle of the basket/paddle apparatuses is based on a simple stirring device. However, operational limitations of such stirrers are such that they cannot provide efficient product/medium interaction or mixing, thus would provide erratic (highly variable) and unpredictable results.
Dissolution method precision – The way I see it
In a recent article titled “Evaluation of In-Situ Fiber Optics Dissolution Method for Compound A Extended Release Tablets” published in the March/April issue of American Pharmaceutical Reviews (Link), the authors described the usefulness of the technique for dissolution testing. The usefulness is described based on method validation parameters such as specificity, linearity, accuracy/recovery, precision, robustness etc.
To me the precision is the most important parameter as almost all the other parameters depend on it, i.e., if the precision is not acceptable then the other method validation parameters will also reflect poorly, thus the method itself.
It is very well accepted and common practice to describe the precision of a method based on Relative Standard Deviation (RSD) or Coefficient of Variations (CV) in percentages. However, in the article (method and intermediate precisions, Tables 5 and 6) authors reported no %RSD (CV%) values. Therefore, the question is how is the precision of the method established? Moreover, why would the authors omit the precision (%RSD) values? Continue reading
Obtaining bio- (physiologically) relevant dissolution results
It is often highly desirable to obtain bio-relevant results as such results increase the confidence and usefulness of the testing. In fact, one should always focus on achieving bio-relevant results as non bio-relevant results are of limited use.
To obtain bio-relevant results one should try to evaluate products using experimental conditions as close as possible to the conditions one would expect during the physiological testing and/or the use of the product.
For the evaluation of in vivo drug dissolution, as conducted based on the bioavailability/bioequivalence studies, it is a common regulatory requirement that products are tested using a standard and common protocol. For example, for the evaluation of IR vs ER products, and any release type in between, the study protocol (physiological environment) remains the same. Therefore, if one wishes to achieve bio-relevant results then one has to conduct in vitro testing using a common set of experimental conditions and these should be product independent as well.
Conducting dissolution studies using product dependent experimental conditions violates this principle, thus should not be considered bio-relevant. To obtain bio-relevant results, testing should be done using a common set of experimental conditions which should also be product independent.
Info
Today, I have added a list of my selected publications on the subject, under the section (sub-heading) of “Useful Lists”. Hope you will find the articles useful.
Drug dissolution testing: Mixing by peristaltic motions vs stirring
One of the requirements for dissolution testing is a mixing mechanism to provide efficient product (tablet/capsule) and medium interactions. Within the GI tract such a mixing mechanism is provided by peristaltic compressions and motions.
To evaluate dissolution characteristics in vitro, one needs to provide a mixing mechanism as well. Question is should the in vitro environment have this mixing/stirring based on peristaltic mechanism as well? The answer is not necessarily yes. The reason being that for any in vitro testing, one tries to simulate an in vivo environment or process, but not to duplicate it. This is one of the basic underlying principles of conducting in vitro testing. There are numerous examples of such practices.
For example, in vitro cell growths (cultures) are usually achieved in simple media not in body fluids. Dissolution tests are conducted using simple media (e.g. buffers). Similarly, controlled temperature environments (baths or cabinets) for testing are maintained, including for dissolution testing, using electronic thermostats with heating elements with or without circulating gasses or water. None of these reflect in vivo environments. The mechanisms to simulate the in vivo environments do not require duplication of the physiological (feed-back) processes where such controls are achieved based on enzymatic-based chemical reactions and circulating physiological fluids. It is important to note that arguments (suggestions) of conducting tests by duplicating physiological environments are usually a reflection of lack of appreciation and experience in the physiological aspect of dissolution testing. Continue reading
IVIVC – Conflict between practices and objective/intent
For a more detailed discussion on this subject along with a description of a simple method for determining C-t profiles, please see the article (The Open Drug Delivery Journal, 2010, 4, 38-47. (Link).
Product dependent dissolution testing – a scientifically invalid practice
It is often suggested that as drug release mechanisms may differ from product to product dissolution tests conditions/methods, therefore, should also be product dependent to reflect these differences in drug release mechanisms. Often, such reasoning is provided for extended-release products and strangely enough not for immediate-release products. For example, recently (Link, Feb. 2011 issue, Q&A section) such an opinion is provided for nifedipine extended-release tablet products. In reality, however, it is not a correct view and is scientifically invalid as well. Continue reading
Selecting an RPM for dissolution testing
All dissolution apparatuses, or perhaps more accurately dissolution testing in general, have serious problems regarding the lack of appropriate and standardized agitation (stirring and mixing) value. Analysts do not know what should be an appropriate rpm (in the case of Apparatuses 1 and 2), flow rate (in the case of Apparatus 4) and dip-rate (in the case of Apparatus 3). This is a big problem. Every analyst sets this (e.g. rpm) based on his or her preference to achieve some desired dissolution characteristics, thus the test loses its usefulness.
Considering this deficiency, a common agitation speed has been suggested using a new spindle (crescent-shaped). It is to be noted that the crescent-shape spindle was chosen for this purpose because Paddle/Basket would not provide appropriate stirring and mixing. These apparatuses have a design/operation problem, which is very well and extensively documented in the literature. I am of the opinion that if one could make a slight adjustment to the Apparatus 1 and 2 by using crescent-shaped spindle then the current problems in dissolution testing can easily and economically be resolved.
A recent article
Title: Limitations of Some Commonly Described Practices in Drug Dissolution Testing and Suggestions to Address These.
… In conclusion, it may be argued that most of the deficiencies/problems of current practices of dissolution may be related to poor hydrodynamics within the paddle and basket apparatuses which also lack relevance to physiological environment. The dissolution testing may significantly be improved if its role may clearly and objectively be established that the tests are to be conducted only to reflect in vivo dissolution characteristics of a product. This clarity of objective will provide an improved basis for selecting appropriate apparatuses and associated experimental conditions. In addition, such an objective will also reduce significant work load by eliminating requirements of repeated IVIVC developments and other physiologically nonrelevant testing. American Pharmaceutical Review, Jan/Feb. 2011. (link)
Paddle and Basket Apparatuses require validation
Current practices of dissolution testing invariably use paddle and basket apparatuses. In fact, describing a dissolution test almost always assumes that the tests been conducted are using these apparatuses.
The main objective of a dissolution test is to evaluate or predict dissolution characteristics of a product in vivo, mostly in humans. Often this link, or predictability aspect, is referred to as in vitro-in vivo co-relationship (IVIVC). Continue reading
Mechanical and/or chemical calibration – why bother?
It is often debated as to which approach is better or more appropriate for calibration or standardization of the apparatuses. It appears that calibration may not be a critical or important step, at present. The reason being, even if the apparatuses (paddle and basket) are adequately calibrated using any of the two approaches, they still would show lack of relevance of results and provide very high variability in dissolution results.
It has been shown from experimental studies and computer simulation modeling that paddle and basket apparatuses provide poor hydrodynamics, thus results obtained from these apparatuses would be of limited value and use. As a results the calibration aspect becomes secondary.
Dissolution testing for products development?
It is often claimed that drug dissolution testing is a useful technique during the product development stage. Does this claim have merit? Let us explore.
A formulator prepares two or more formulations/products having different dissolution rates using commonly described dissolution test conditions. How would the formulator decide which product can be tested in humans? For this purpose the formulator needs to have some confidence in the predictability of the dissolution test for the behaviour of a product in humans. It is well known that current practices of dissolution testing do not provide such predictability, thus the testing cannot be used for product development. There are examples showing that products having differences in vitro results, provides overlapping in vivo results.
Then why do people suggest the use of dissolution testing for product development. Apparently, the suggestion is correct but not its interpretation. In principle, it is correct that dissolution tests should be reflective of in vivo results. However, success will depend on how a dissolution test is conducted and what type of instrument/apparatus is used. Presently, people invariably assume that dissolution testing means conducting a test using paddle and basket apparatus. The missing link here is that these apparatuses have never been validated to provide relevant in vivo conditions (environment) to predict in vivo results? Obviously, these apparatuses cannot provide relevant in vivo results. It is like saying that can a distance from point A to B, 1000 miles apart, be travelled in an hour by road. Of course, yes, but we need a car which would be able to run at a speed of 1000 miles/hour. Objective is fine but the practicality of achieving the objective is not. This is exactly what is happening with the current practices of drug dissolution testing, i.e., the objective is fine but means (paddle and basket apparatuses) to achieve the objective is not.
Thus, the common practices of dissolution testing fail to provide relevant in vivo results and will not provide relevant results at the product development stage.
Lack of success in IVIVC for BCS class II drugs
The other day someone indicated that even products of drugs from BCS class II (low solubility and high permeability) have not shown successful IVIVC. These drugs, at least in theory, provide the best case scenario for successful IVIVCs. The question was then asked what may be the reason for such a general lack of success.
For any successful IVIVC one needs to conduct dissolution tests by mimicking the in vivo environment as closely as possible. This is usually done by conducting a dissolution test using water or aqueous buffers having pH in the range of 5 to 7 maintained at 37C. These conditions represent the GI tract (intestinal) environment.
On the other hand, the tests are conducted mostly using paddle and basket apparatuses to simulate mixing and stirring environment. Unfortunately, the stirring and mixing environment of these apparatuses lack simulation of the in vivo environment. In fact, these apparatuses almost provide no stirring and mixing. Therefore, because of this mismatch, one should not expect successful IVIVC. For successful IVIVC, one requires an efficient (gentle but thorough) stirring environment. One such possibility to address this issue may be the use of crescent-shape spindle. For further discussion on the use of crescent-shaped spindle one may search this site or literature in general.
In short, one should not expect success in developing IVIVC using paddle and basket apparatuses.
Drug dissolution testing for monitoring batch-to-batch consistency – an illusion?
It is often described that one of the purposes, or perhaps the only purpose, of drug dissolution testing is to monitor batch-to-batch consistency in manufacturing processes. I believe that this view is described to maintain the use of dissolution testing based on paddle and basket apparatuses. This view appears to have been out of a frustration due to a lack of success with dissolution testing regarding its relevance to a product’s in vivo performance.
The question remains, can the testing be used for the consistency check? The answer appears to be a NO. The testing cannot be used for consistency check in particular using paddle and basket apparatuses. The reason being that for monitoring consistency of a product or process, the consistency (reproducibility) of the test itself must be established and known first. Unfortunately, consistency (reproducibility) of the testing based on paddle and basket apparatuses have never been established or available. There are literature reports available which provide a measure of expected variability in dissolution testing. The reported variability values in terms of RSD can be as high as 37% using these apparatuses, with the apparatuses working as expected and meeting the USP specifications. Such a high variability in testing instruments is not usually acceptable, as the test would not be capable of providing stringent quality control standards for pharmaceutical products where generally desired variability (RSD) of 10% or less is expected or desired.
Thus, dissolution testing based on paddle and basket apparatuses may not be used for batch to batch consistency checks.
PVT – Repeated attempts to convince would not make it right or good.
PVT (Performance Verification Test) is frequently described as necessary to assess the performance of dissolution apparatuses (paddle and basket). Interestingly, the test quite often fails i.e., test results often fall outside the expected range, without any known reason or cause.
Commonly described reasons/causes are; worn-out ball bearings, loose motor belts, misalignment of spindles or vessels, inaccurate gap between bottom of spindle and base of vessel, lack of straightness of spindle rods, wobbling, vibration in the instrument and/or around its surrounding, high/low humidity effecting tablets, inappropriate de-aeration of medium, inaccuracy in measured rpm, variations in vessel dimensions, mismatch of vessels from different suppliers, not using vessels from the instrument supplier, use of plastic vs glass vessels, using scratched or not clean vessels, not withdrawing a sample from an appropriate position, not appropriately dropping the tablet or pouring the medium in the vessels, lack of an analyst’s training, in addition, any combination/permutation of these reasons.
Most interesting is the fact that there has been no experimental evidence available in support of these claims i.e., there is no experimental data available to indicate that these aberrations provided results outside the expected range. To rationalize its continued use, supporters of the PVT maintain the claim that failures indicate potential deficiencies or aberrations, but how?
A dissolution test as a monitor of batch-to-batch consistency in manufacturing:
The primary purpose of the dissolution test is to distinguish between acceptable and unacceptable batches of a product for human use. However, it is now widely recognized that current practices of dissolution testing may not be used for such purposes, i.e., for bio-relevancy purposes.
Therefore, rather than addressing the underlying deficiencies and improving upon these, the test now commonly propagated as a measure/monitor of batch-to-batch consistency of the manufacturing process. It is not clear which element(s) of manufacturing process(es) the test is linked to and how such a link has been established. In addition, there is a lack of validation of an appropriate link of dissolution to manufacturing. In the absence of such validation, it is not possible to describe this test as performance or quality control/assurance test.
The current practice of dissolution testing as a QC test may be equated to the installation of a sophisticated digital camera to take a picture of every finished car coming out on a assembly line. As long as the pictures are consistence from car-to-car, a car’s performance and quality may be assumed “assured”. However, as the picture and performance are not linked, there is no guaranty that an acceptable picture in reality will reflect an acceptable performance of the car and vise versa.
Similarly, as a dissolution test is not linked to the performance of a product, acceptable dissolution results may not reflect acceptable performance of the product and vice versa.
A twist in the objective of drug dissolution testing.
Drug dissolution testing is an important and critical technique for developing and evaluating the quality of a drug product based on its release characteristics in vivo i.e., in the human GI tract, in particular the small intestine.
Often dissolution studies are conducted using paddle and basket apparatuses. However, testing using these apparatuses has shown significant frustrations in obtaining relevant results with acceptable variability (reproducibility).
Significant literature is available describing the flaws of these two apparatuses. One such flaws is that, although generally assumed, these apparatuses have never been validated for their intended purpose i.e., obtaining relevant and reproducible results.
Even though these flaws are generally recognized, these apparatuses are in use as a “tradition” because these are the ones most commonly suggested and employed in the past. Another reason of their continued use is an apparent and unfortunate twist in the objective of the dissolution testing. That is, rather than evaluating dissolution characteristics of a product, it is often suggested to establish the experimental conditions that show the desired dissolution characteristics. Thus, there is a large waste of human and financial recourses in developing drug and product dependent procedures.
The practice of obtaining or showing the desired and product dependent dissolution results has no purpose other than rationalizing the continued use of paddle and basket apparatuses.
To conduct appropriate dissolution studies, one needs to focus on the true objective of the testing i.e., to observe drug dissolution/release characteristics of a product in vivo. With this objective things will start to fall in place. This will allow the analyst to use more efficient apparatuses and simple experimental conditions to obtain useful results.
Level A IVIVC – Impractical and unreasonable expectations
An IVIVC (in vitro-in vivo co-relationship) is a terminology commonly used in the area of drug dissolution testing desiring relationship of in vitro (drug dissolution) results with in vivo characteristics such as drug levels in humans.
As the in vitro results are generally expressed as cumulated percent drug release, thus these profiles (results) are difficult to compare with in vivo results which are reported in concentration units. In addition, in vitro results only reflect release (dissolution) characteristics of a product while in vivo results reflect combined effect of drug dissolution and absorption/elimination characteristics. To achieve appropriate comparison, either the in vitro dissolution results are manipulated (mathematically) using drug absorption/elimination characteristics to predict blood levels and compared with actual/observed drugs levels or by extracting in vivo dissolution results from actual drug levels in humans and to compare with the observed in vitro results. The first approach is known as a convolution technique and the second as de-convolution. One of these techniques of data manipulation would be required to make the results comparable. Continue reading