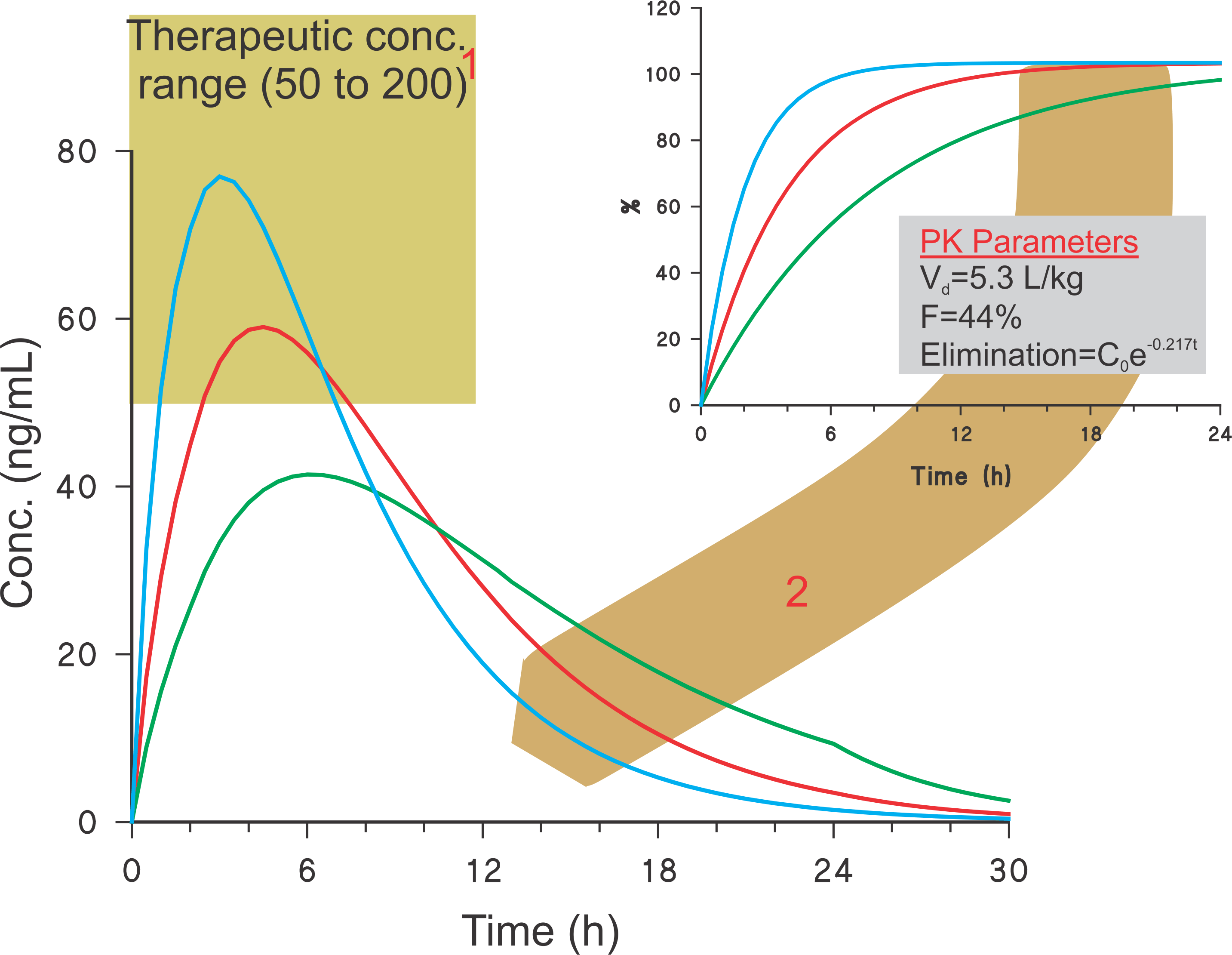
Estimation of blood levels – Example: 120 mg diltiazem ER capsules
Defining a dissolution apparatus
In general an apparatus means a machine having a specific function. In our case, a dissolution apparatus means “a machine which may be used to determine dissolution characteristics (function) of a drug product such as a tablet or capsule”. This function (dissolution) can only be achieved if the machine is able to provide thorough but gentle (low rpm) stirring and mixing within a vessel. Another way of saying the same thing is that the machine should provide thorough but gentle product/solvent interaction at low rpms, ie, less than 100.
 The reason the machines shown above may not be considered as dissolution apparatuses is that they do not provide an appropriate and required product/solvent interactions, thus drug dissolution. All these machines required very high speed (rpms) for mixing. At lower rpms the test products often lie around stagnant, thus incorrectly indicating limited or no dissolution.
The reason the machines shown above may not be considered as dissolution apparatuses is that they do not provide an appropriate and required product/solvent interactions, thus drug dissolution. All these machines required very high speed (rpms) for mixing. At lower rpms the test products often lie around stagnant, thus incorrectly indicating limited or no dissolution.
A critical requirement for a dissolution apparatus is that it should be capable of providing stirring and mixing at low rpms and it must also avoid stagnation of the test product.
Developing and validating a dissolution method – suggestions for simplification
Method development and validation is a critical and important part of drug dissolution testing and is required for an appropriate product evaluation. It is a common practice and often time confusing and frustrating exercise. In addition, these exercises are often described by different names such as developing QC/QA, bio-(relevant) and/or discriminatory methods. In additions, these method development exercises are further complicated by requiring different types for each drug and product. In fact, a single type of product of the same drug, in particular ER, can have multiple methods.
On the other hand, however, if the methods described in literature are critically evaluated, it would be quite obvious that there are not significant differences in these methods. The majority of the methods described in literature are based on USP apparatus 1 (Basket) or 2 (Paddle) set mostly at 100/75/50 rpm with water or some form of buffer, having a pH in the range of 5-7 as a medium. This experimental part is followed by usual method validation steps. Confusion and frustration arise from the question of how to select a particular set of experimental conditions (apparatus, rpm and medium). Unfortunately, there is no rationale or scientifically valid approach available for selecting the required set of experimental conditions. Formulators or analysts are often expected to make their own judgment calls, which causes the confusion and frustration.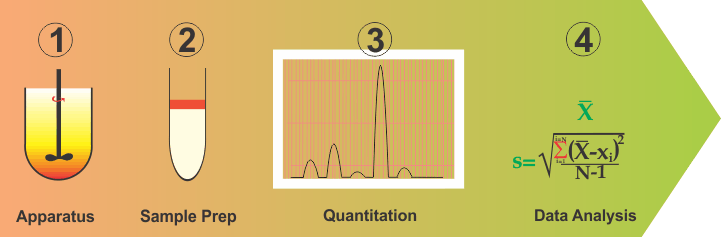
Although, complex and difficult as this situation may be, one should be able to address this problem by simplifying and breaking down into parts the method development and validation exercises. In this regard, one can break this down into four distinct parts: (1) Apparatus containing medium; (2) Sampling or cleanup step; (3) Quantitation and; (4) Data analysis (or validation). Continue reading
Info
Useful Lists Section – Updated (Sep 22, 2011). (Link)
Multiple vs Single-Point Tolerances
It often puzzles people as to how one should select one approach over the other. It is often considered that for quality control (QC) purposes one may use single-point approach, while during product and/or method development stages one may use multi-point sampling approach. The single point approach is often derived from multi-point sampling used during the product development stage. It is important to note that for ER products usually there are no single-point tolerances, even for QC purposes. Should, or do, these approaches provide different outcomes? For example, with the multi-point sampling approach dissolution may be measured at 5, 10, 15, 20, 30, 45 and 60 minutes, and for QC purposes a single sampling time of say 30 minutes may be chosen. The dissolution results at 30 minutes should exactly be the same whether one uses multi-point or single point approach as dissolution is property of the product which remained the same. Continue reading
Faster Dissolution Results Do Not Necessarily Mean A Loss Of Discriminatory Power
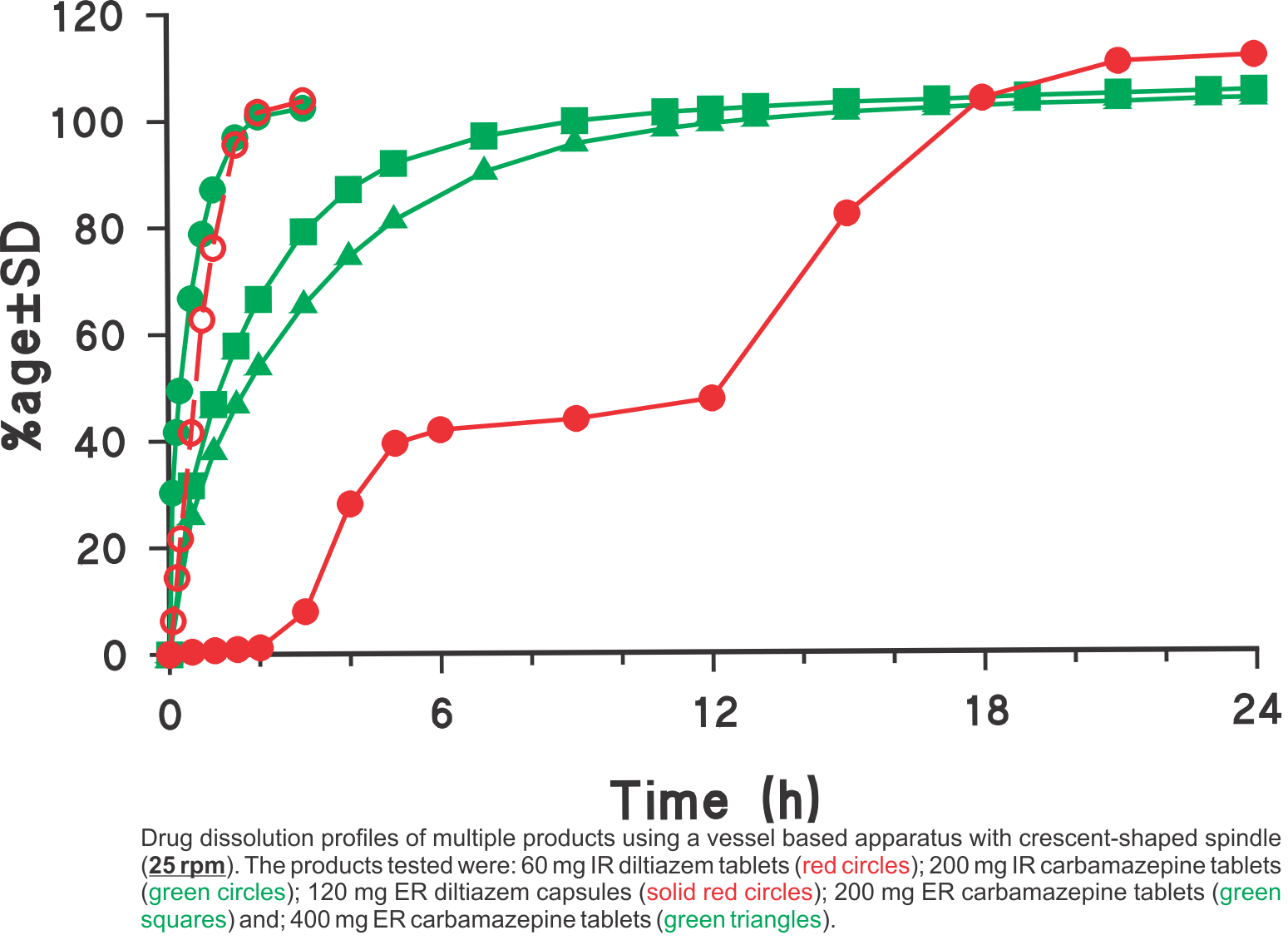
Often it is assumed that if a test provides faster dissolution, then its discriminatory ability may be diminished or compromised. Unfortunately, this is an incorrect view. In this regard, it is invariably assumed that the slower results obtained using Paddle/Basket apparatuses are the reference, or the most discriminatory, and any increase in dissolution rate should by default be considered as less discriminatory. 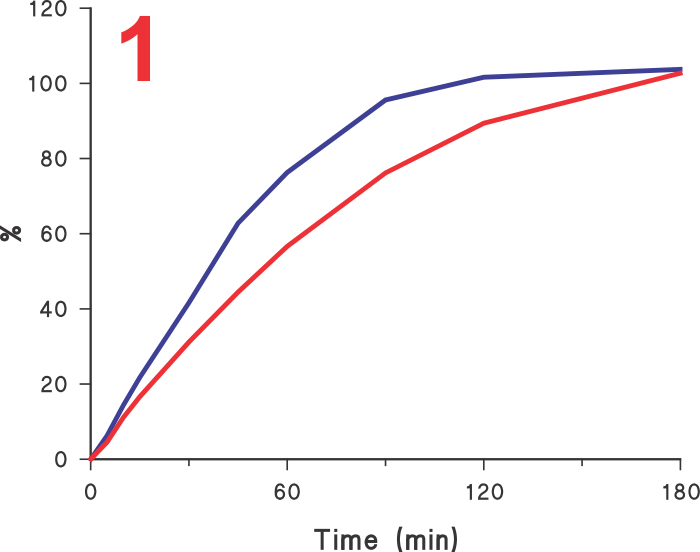 However, before one considers this comparison, it may be important to decide which results are the correct ones, the slower one obtained using Paddle/Basket or faster ones using any other approach (higher rpm, different apparatus etc). Looking at the Figure 1 let us assume that the lower profile is obtained using the Paddle apparatus and the upper using some other experimental condition, should the method which produced the upper profile be considered less discriminatory or less desirable? It is quite possible that in fact the upper curve may be a more accurate representation of the characteristics of the product than the lower one. If so, then the results obtained using Paddle, even slower, should be considered as an incorrect representation of the product.
However, before one considers this comparison, it may be important to decide which results are the correct ones, the slower one obtained using Paddle/Basket or faster ones using any other approach (higher rpm, different apparatus etc). Looking at the Figure 1 let us assume that the lower profile is obtained using the Paddle apparatus and the upper using some other experimental condition, should the method which produced the upper profile be considered less discriminatory or less desirable? It is quite possible that in fact the upper curve may be a more accurate representation of the characteristics of the product than the lower one. If so, then the results obtained using Paddle, even slower, should be considered as an incorrect representation of the product.
This is in fact the case here. The profiles shown here are of 60 mg diltiazem IR tablet products using Paddle (75 rpm, lower curve) and crescent-shape spindle (25 rpm, upper curve). The reason for the slow rate of drug release from tablets using Paddle, even at 75 rpm, is that the tablets are stagnant at the base of the vessel (Figure 2) and there is no, or limited, tablet-medium interaction or drug dissolution from the bottom of the tablet.
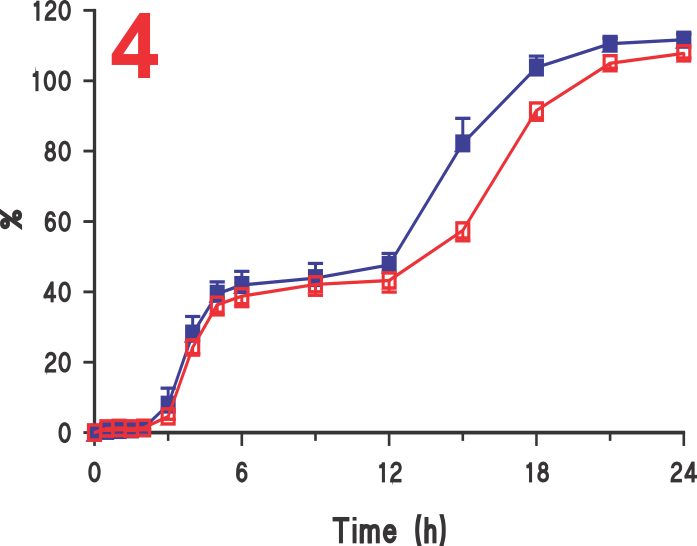
On the other hand, as the tablet moves with crescent-shape spindle, the tablet-medium interaction is from all sides of the tablet and thus faster dissolution with corresponding higher results (Figure 3). Similar, behaviour was observed with beads from a capsule product. In this case, a 120 mg ER diltiazem capsule product was analyzed (Figure 4). Again, lower (slower) drug release was observed using Paddle at 75 (Figure 5), while higher drug release was observed using the crescent-shape spindle (Figure 6). It can, therefore, be concluded that lower dissolution rate using Paddle may not reflect a better discriminatory ability but is a poor reflection of product-medium interaction, thus false dissolution characteristics of a product. For further discussion on the subject of poor product-medium interaction, and poor dissolution using Paddle apparatus please see the publication (link). 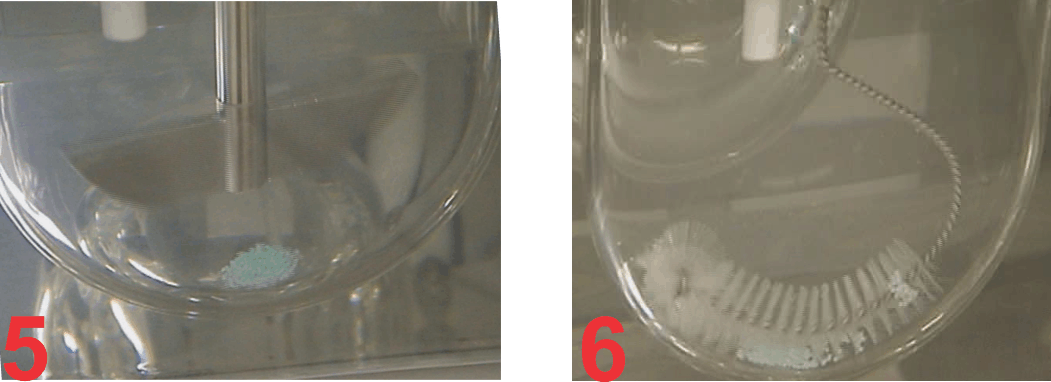
Developing a Dissolution Method – What is Required?
Developing a dissolution method requires an apparatus with associated experimental conditions capable of providing an expected outcome, in this case dissolution characteristics of a reference product. The dissolution characteristics of the reference product must be known and established independently. As, at present, there is no reference product available with known or accepted drug release characteristics, one cannot develop a dissolution method, in the true sense of the concept and/or practice of analytical chemistry.
However, this limitation may be addressed by using the concept of relative dissolution. i.e. developing a method (apparatus and associated experimental conditions) which will be capable of differentiating dissolution characteristics of approved IR and ER products of the same drug. Also, this differentiation in dissolution results should be achieved within the dosing interval of these (IR and ER) products. The practice of such an exercise will be called “dissolution method development” and such a method should be used for determining the dissolution characteristics of a test product. As a rule, an appropriate dissolution method has to be product independent.
It is important to note that a dissolution method cannot be developed using a product which is being developed. One should be careful in reading and interpreting the literature in this regard.
Are soluble and poorly soluble drugs classifications appropriate?
It is often described in literature that method developments for poorly/sparingly soluble drugs pose special challenges, thus, may require a more careful and elaborate approach. Such views do not appear to be convincing.
First of all a dissolution test is conducted for a product (tablet/capsule) not for a drug. Therefore, if the product (formulation/manufacturing) remains the same, containing either a freely or sparingly soluble drug, then the same method should perform well. However, the difference will be in the monitoring (quantitation). If an appropriate medium is not used, then quantitation would either be erratic or not possible for low solubility drugs.
On the other hand, often quantitation does not cause problems, because as a fundamental requirement, a dissolution test should only be conducted under a sink condition i.e. the expected amount of drug from the product must be freely soluble in the medium. This “sink” condition is established prior to method development. Therefore, it is important to note that high or low solubility relates to general aqueous solubilities of drugs, not to dissolution medium or testing. For a dissolution test a drug must be freely soluble, therefore, both (high and low solubility drugs) are equivalent for dissolution testing.
 The question is then why do the products of these two types of drugs often behave differently in dissolution testing so that they required a different classification or different approaches. The reason is that it is because of the poor interaction between the product (disintegrated) content and the dissolution medium. Due to poor hydrodynamics within dissolution vessels, paddle and basket, the product contents tends to accumulate at the bottom. This accumulation is known as “cone” formation (as shown in the picture). Often such pronounced “cones” are not observed with the basket apparatus, but similar accumulation/settling of the product contents at the bottom of the vessel is present. Depending on the size of the “cone”, the release of the drug from the “cone” will be different, mostly slower and erratic than expected. The size and shape of the “cone” will depend on the excipients (nature and amount). Bulkier and larger amounts of excipients would have a greater impact. In short, it is not a problem because of the low aqueous solubility of a drug or the product itself, but poor hydrodynamic (stirring and mixing) in a dissolution vessel whether it is paddle or basket apparatus. Unfortunately, this is an inherent weakness of the paddle and basket stirrers and cannot be resolved.
The question is then why do the products of these two types of drugs often behave differently in dissolution testing so that they required a different classification or different approaches. The reason is that it is because of the poor interaction between the product (disintegrated) content and the dissolution medium. Due to poor hydrodynamics within dissolution vessels, paddle and basket, the product contents tends to accumulate at the bottom. This accumulation is known as “cone” formation (as shown in the picture). Often such pronounced “cones” are not observed with the basket apparatus, but similar accumulation/settling of the product contents at the bottom of the vessel is present. Depending on the size of the “cone”, the release of the drug from the “cone” will be different, mostly slower and erratic than expected. The size and shape of the “cone” will depend on the excipients (nature and amount). Bulkier and larger amounts of excipients would have a greater impact. In short, it is not a problem because of the low aqueous solubility of a drug or the product itself, but poor hydrodynamic (stirring and mixing) in a dissolution vessel whether it is paddle or basket apparatus. Unfortunately, this is an inherent weakness of the paddle and basket stirrers and cannot be resolved.
In conclusion, classification of high (freely) or low (sparingly) solubility drugs do not appear relevant for drug dissolution test purposes.
Choosing an Apparatus: Paddle vs Basket
A commonly asked question is how should one choose between Paddle and Basket apparatuses when selecting an apparatus? In short there is no clear cut answer, in particular based on a scientific merit or reasoning.
It is important to note that both of these apparatuses have been shown to provide highly variable and unpredictable results. Furthermore, the results obtained using these apparatuses often lack a link to physiological or in vivo characteristics of the test products. These apparatuses usually provide two different sets of results for the same product under similar operating conditions. Thus, it would be impossible to know which one is reflecting the actual/true dissolution behaviour of the product. Between the two, the Basket apparatus appears to provide more variable results than the Paddle apparatus.
It appears that traditional practices/views, rather than scientific merit, are used in selecting the apparatuses. For example, it is commonly suggested that the Basket apparatus may be preferred for products which may float in the dissolution vessels. On the other, hand, such floatation may be controlled with the use of a “sinker” if one prefers to use a Paddle apparatus. Eventually, it boils down to the personal preference of an analyst, as to what his or her expectations are for the dissolution behaviour of the test product.
In short, current practices are to choose an apparatus which would provide desired dissolution results (behaviour) of the test product. How useful or relevant would such results be? This remains an open and debatable question.
A Concern Which Requires Urgent Attention
Drug dissolution testing is often described as a quality control tool for the assessment of pharmaceutical products, such as tablet and capsule, by establishing batch-to-batch consistency in their production (see). The question is what quality and consistency here one refers to? Saying it in another way, if an analyst conducts a dissolution experiment and obtains certain results, how these results are linked to the quality of the test products. If establishing repeatability/reproducibility/consistency of a product and/or results is the objective, then why can this be achieved ONLY by conducting a dissolution test , when other tests can also be done, e.g., disintegration or grinding (to achieve consistent fine powder). If a product disintegrates or ground to fine powder then expected batch-to-batch consistency is established. There is actually no need to conduct a dissolution test for such a purpose. Manufacturers and regulators have to consider this aspect carefully as to why a dissolution test is necessary to establish batch-to-batch consistency.
On the other hand, if a dissolution test as a solution formation test is needed then perhaps one can do a test using a 50/50 solution of water and ethanol with stirring for half an hour at an rpm of 150 for all products. It is not necessary that the entire drug has to be released or dissolved as long as the results are consistent and reproducible. Such a test can also meet the requirement of a consistency test.
Moreover, a dissolution test is suggested as a consistency test, however, there appears to be nothing consistent about the test itself. For example, one may use any apparatuses (mostly paddle or basket), with any rpm (mostly 50, 75 or 100) using any volume of medium (mostly 250, 500, 900 mL or 1L) having any pH (mostly 1, 4.5, 6.8) of any strength (mostly 0.01 to 0.1M) and having any solubilizing agent (mostly SLS). Further, a dissolution test can be run for any duration of time from 15 minutes to 24 hours. There are no criteria in choosing a consistent experimental condition other than choices based on the discretion of a formulator/analyst to achieve certain DESIRED dissolution characteristics of the product.
In short, a dissolution test as it is conducted or suggested currently does not appear to provide scientific or logical support so that it can be considered as a test to monitor batch-to-batch consistency of pharmaceutical products.
So why is a dissolution test conducted? The only reason the test is to be conducted is to assess in vivo dissolution characteristics of a product. This is a very important concept/requirement which somehow has been overlooked and requires urgent attention.
Considering IVIVC – requires caution
IVIVC is commonly referred to as having an in vitro dissolution method which is able to relate dissolution or absorption properties of a drug in vivo, mostly in humans. It is further assumed that an analyst will be using a paddle or basket apparatus for developing such IVIVC. On the other hand, it is interesting to note that the use of paddle and basket apparatuses has never been validated for establishing IVIVC i.e. whether, or not, these apparatuses are capable of providing relevant in vivo results. Successful examples of such IVIVC are rare, if any. More recent literature highlights that lack of an appropriate stirring/mixing environment within dissolution vessels for these apparatuses may NOT properly reflect in vivo (or bio-relevant) environment thus demonstrating their inability to provide IVIVC.
Therefore, when considering developing IVIVC, or evaluating published literature/studies in this regard, one should be cautious as conclusion drawn could be misleading and/or errounous.
Clarification:
I often receive comments and queries regarding one of my publications on the subject of IVIVC and determining C-t profiles. The method for establishing C-t (blood conc.-time) profiles described in the publication [Link] appears to be quite popular and acceptable. However, it appears that readers are missing a critical aspect of the publication, i.e., the use of crescent-shaped spindle for obtaining the dissolution data which are used for calculating the C-t profiles.
For successful C-t profiles development, it is critical that the in vitro dissolution test method employed must be capable of producing in vivo relevant, or bio-relevant, dissolution results. Unfortunately, as paddle and basket apparatuses are not capable of providing bio-relevant results, the methodology described in my publication may be of limited use or help for the data generated with paddle and basket apparatuses.
Therefore, please make sure that when you determine the C-t profiles, you are using a bio-relevant dissolution tester, and the associated experimental conditions, to generate in vitro dissolution results.
Currently suggested dissolution testers/methods may not be capable of determining dissolution characteristics of drug products.
It is common understanding and practice that an appropriate dissolution test is to be developed for a particular drug product when the product itself is being developed. The rationale being that the product development stage provides sufficient variety of formulation/manufacturing differences along with in vivo (bioavailability/bioequivalence) data to establish the relevance and validity of the proposed dissolution test. The choice of experimental conditions (such as apparatus, rpm, medium, pH, etc) which fit the product’s behaviour the most be chosen as the best or most appropriate dissolution test for future use. If one method may not fulfil the need or requirement, as commonly happens, then two or more methods for the same product may be suggested such that one (simpler) would be used as a QC test and the other (somewhat complex) as bio-relevant. In short, while products are developed, dissolution test(s) are being developed for the product evaluation. Continue reading
Drug Dissolution Testing Using Simple and Common Experimental Conditions
Dissolution profiles were generated using the USP vessel apparatus with crescent-shaped spindles set at a rotation speed of 25 rpm in all cases. The media used was 900 mL water for diltiazem and 900 mL water containing 0.5% sodium lauryl suphate (SLS) for carbamazepine products, respectively. The SLS was added to provide the needed sink condition. Using the suggested experimental conditions, all one has to do is to provide an appropriate dissolution medium (water with or without a solubilising agent e.g. SLS) so that the expected amount of drug is soluble in the medium.
This single method/approach was employed for analyzing different types of products: tablets, capsules, IR and ER products having high water solubility (diltiazem) and low solubility (carbamazepine). As these experimental conditions are commonly used and simple, the method may easily be transferred to a QC test along with bio-relevant support of the testing environment. For further explanation and discussion on this topic please refer to the publication (link).
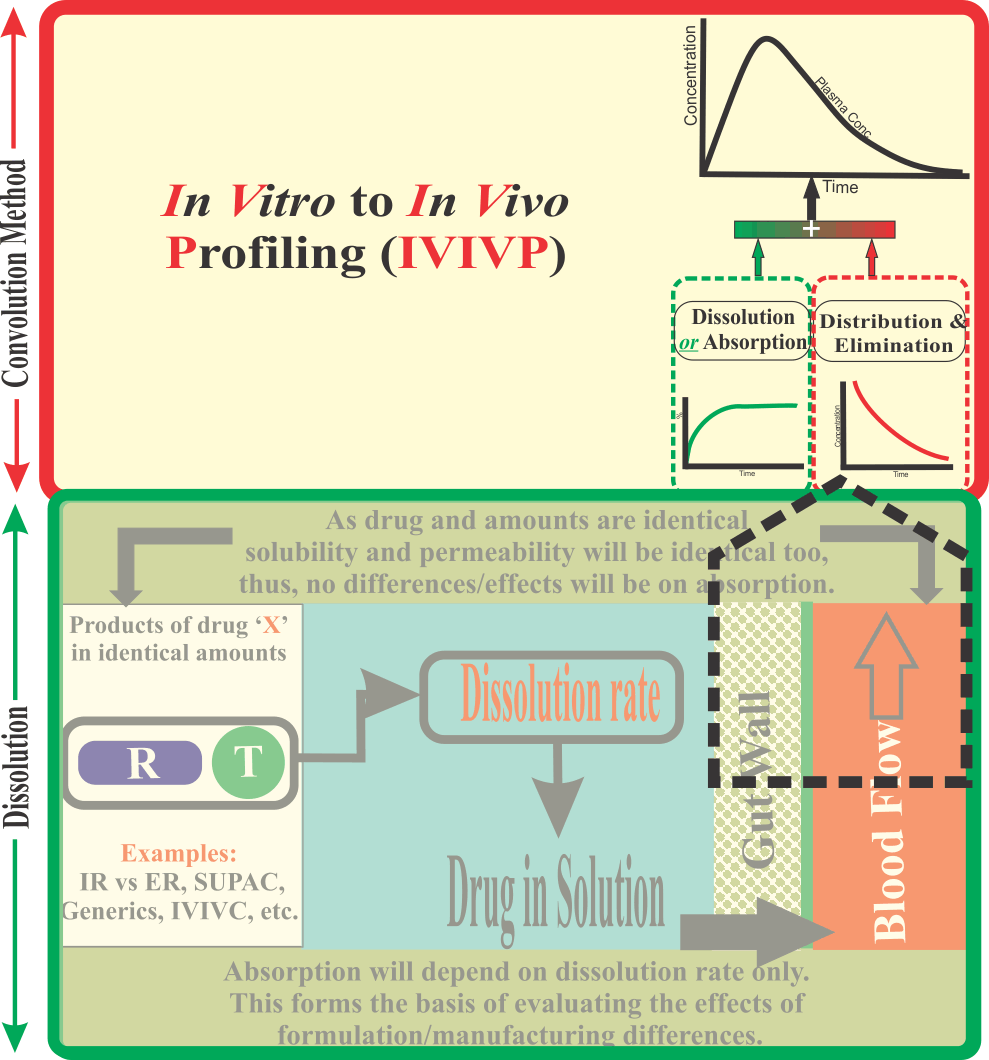
In Vitro-to-In Vivo Profiling (IVIVP)
Has BCS (Biopharmaceutics Classification System) been a Futile Exercise?
It appears so.
BCS is an approach which has been propagated to reduce the burden of pharmaceutical product evaluations and their regulatory approval using drug dissolution testing instead of in vivo (bioavailability/bioequivalence) testing.
It should be noted that in reality the BCS concept was introduced to facilitate success in establishing IVIVC, which in general had shown poor, or no, success.
The underlying principle of BCS is that if drugs are classified into classes based on their aqueous solubilities and absorptions or permeabilities through the GI tract, then establishing IVIVC may be possible. It is, therefore, important to note that the concept of BCS was introduced to increase the chances of IVIVC success.
For BCS, drugs are divided into four classes having the characteristics of: (I) High solubility and high permeability; (II) Low solubility and high permeability; (III) High solubility and low permeability; (IV) Low solubility and low permeability. As per BCS approach, for drugs which would fall in the low permeability category i.e. classes III and IV, it would be unlikely to achieve successful IVIVC as drug dissolution testing does not relate to permeability. Thus, success of IVIVC may not be possible for the drugs of these classes. The drugs in class I are also considered as poor candidates for a successful IVIVC as these drugs would often dissolve so fast that they may overwhelm the absorption system. Therefore, the drugs in the class II (low solubility and high permeability) and drugs of class I, where release would be manipulated so that drugs appear as slow dissolving (e.g. extended-released type) would have the potential to achieve successful IVIVC. It is important to note that in principle BCS would support potential success of IVIVC with only class II type drugs. However, reported success of IVIVC for drugs in class II has also been limited, (perhaps because of mismatch of in vitro and in vivo environments, see link) and one should be cautious when describing the BCS as a success or useful practice. Thus, the extension of BCS in developing IVIVC and its usefulness in regulatory environment should be considered with care.
On the other hand, there are Guidances available which suggests that the use of the BCS concept for bio-waivers, meaning products may be evaluated using in vitro drug dissolution tests only without in vivo testing. First of all, these Guidances are applicable for products of drugs in class I only, where generally it is recognised that the achieving IVIVC would be highly unlikely based on the BCS concept as explained above. Moreover, there are other conditions as well which these drugs and their product must also meet. For example, products must be of immediate release type and the products must also release the drug very quickly, generally in less than 30 minutes. In essence, the products for which bio-waiver may be considered should be such that they should have drugs which would be released and dissolve quickly. The assumption here is that the human body will consider these products equivalent to solution products. It is obvious from this discussion that the BCS which intended for developing IVIVC plays a limited role in describing bio-waiver criteria for this particular class of drug where no IVIVC is expected.
It may, therefore, be concluded that BCS had been of limited use in facilitating IVIVC, application of dissolution testing in lieu of in vivo testing and/or reducing regulatory burden.
Dissolution method development – a practice which causes confusion and hinders in product evaluation
In my opinion, current practices of method development have not only caused the biggest confusion in the industry but also hindered in an appropriate evaluation of drug products using dissolution (release) testing itself.
Current practices of method development suggest that an analyst is to “fish” (seek) experimental conditions, such as choice of an apparatus (paddle/basket), rpm, buffer, pH to establish UNKNOWN dissolution characteristics of a product. Normally, it is suggested that an analyst should have a number of formulations with presumed dissolution characteristics and the analyst should seek experimental conditions which would reflect his/her “presumed” product characteristics. Such practices are often also referred to as developing “discriminating” and/or “lot-to-lot consistency check” methods.
Similarly, the above mentioned practice of “fishing” (seeking) experimental conditions will be considered as developing a bio-relevant method if the analyst tries to match dissolution results with in vivo results (bioavailability/bioequivalence).
Therefore, in practice, an analyst would never know the true product dissolution characteristics but would select experimental conditions which would fit his/her expectations. It is therefore, critical to understand that as it stands now, an analyst or a product developer will never know the true dissolution characteristics of its product. Each and every analyst/product developer is occupied with “developing” dissolution methods which in reality should not be their assignment. Their objective is to develop a product based on its dissolution characteristics, using a standardized and well accepted dissolution method. This is similar to an analogy in which each and every laboratory would be busy in developing their own thermometers and weighing balances to be used for monitoring temperatures and weighing substances, respectively.
It is therefore, essential to note that the pharmaceutical industry requires a standard dissolution tester along with its associated experimental conditions capable of providing dissolution characteristics of pharmaceutical products. The crescent-shape spindle has been developed with these thoughts in mind, which appears to offer a powerful solution to the current confusion and avoids the unnecessary practices of method development.
Concept of “quality” in drug dissolution testing
In the drug dissolution testing area, a reference to “quality” of a product (table/capsule) is frequently made. For example, a drug dissolution test may be considered as a quality assurance and/or control test. More recently use of the term has been extended to the concept and practice of “quality by design” or QbD. It is obvious that to achieve or monitor “quality”, one needs to define it as an achievable objective or goal.
In general terms, “quality” is a subjective term for which each person or sector has its own definition. For example, certain variations in shape, size or color of a tablet may be an indication of poor quality of product for some but for others it may be normal and expected. In the literature, commonly “quality” refers to “fitness for use” or “conformance to requirements” according to Joseph Juran (considered father of the QbD concept) and Philip Crosby, respectively. However, in technical usage such as for the assessment of pharmaceutical products, these definitions of “quality” may be translated into a property of a product which fulfils stated or implied needs by establishing it as “fit for use”. The terminology of “to fulfil stated or implied needs” appears to be the most critical in this regard, i.e, one has to establish the need before even defining the quality.
Therefore, before using dissolution testing for “quality” assessment purposes (or its control/assurance), one first has to describe what is the stated or implied need here. Until and unless one cannot define or establish a need or use (for the testing) one cannot establish the quality. What is the need or use of a drug dissolution test? Conducting a dissolution test is not a need by itself. However, it is the ability (test) to fulfil a need of determining drug dissolution in human GI tract from pharmaceutical products. The need is determining of drug dissolution in human GI tract. This particular needed characteristic reflects upon the “quality” of the product. How it is to be determined and controlled or assured comes later, which is the “fitness for use” characteristics. In this regard, both, i.e., the use of bioavailability/bioequivalence (BA/BE) testing/studies and drug dissolution testing are two different types of tests to fulfill the same need and establishment of “fitness for use” criteria. One reflects the in vivo evaluation while the other in vitro but they both fulfil exactly the same need. One may argue about the differences in the strength of these two types of tests, but one has to recognize that they both fulfill the same need. Once the need is established, then one has to establish tolerance (specifications) for “fitness to use” around the needed characteristics, so that this need may be fulfill consistently. For further discussion on the evaluation of drug release (dissolution) both in vitro and in vivo, please see the post.
In short, therefore, “quality” here is referred to as fulfilling a need of determining the drug release (dissolution) from a product in the gastrointestinal (GI) tract, which is quantitatively measured to establish the characteristic of “fitness for use” by a drug dissolution test.
Apparatus Calibration or Performance Verification: Misleading Conclusions and False Comfort
In a recent issue of Dissolution Technologies, in an article titled “Overview of Dissolution Instrument Qualification, Including Common Pitfalls” the authors started the article with a statement (or quote) of the claim that “For almost fifty years, the pharmaceutical community has been relying on dissolution data as an indication of drug product performance. Effective qualification of the dissolution apparatus is critical to the value and integrity of these data”.
The above mentioned statements can be misleading and may provide false and erroneous comfort for a drug product performance using instrument qualifications (IQ) as its basis, as explained below: Continue reading
In Vivo vs In Vitro Bioequivalence
In vivo bioequivalence, or simply bioequivalence, is commonly referred to as an evaluation study conducted to establish equality of mostly two oral products such as tablet or capsule. Equality of two products (test vs reference) is established by comparing their blood drug concentration-time (C-t) profiles. The reason for selecting C-t profiles for such comparison is that as therapeutic effects depend on drug concentrations in blood i.e., if two or more products provide similar C-t profiles then they will provide similar therapeutic effects as well, thus they will be considered therapeutically bioequivalent, or simply bioequivalent. [Continue …]
Lack of faith in obtaining physiologically relevant dissolution results. There is a reason for it.
It is often stated that in vitro drug dissolution testing may never be able to predict the physiological outcome as the environment, and processes within, may be too complex and variable to be adequately reproduced in vitro. This prevents to achieve adequate and bio-relevant dissolution results. This belief may not reflect the reality and appears to lack any experimental evidence.
The belief appears to be based on dissolution results obtained using mostly paddle and basket apparatuses. Interestingly, it has been shown repeatedly that these apparatuses poorly mimic physiological environment (e.g. see), which is required for dissolution testing. It is, therefore, should be expected that these apparatuses would not provide physiologically relevant results. In addition to a lack of physiological relevancy, it has further been shown that hydrodynamics within dissolution apparatuses (paddle/basket) is such that these apparatuses should provide highly variable and unpredictable results [link]. It is, therefore, safe to assume that it is not the difficulty and complexity of reproducing a physiological environment in vitro but the choice of dissolution apparatuses which appears to have caused the lack of success. Recent studies using the modified spindle (crescent-shaped) which addresses the artifacts of the paddle and basket apparatuses, appear to provide choice of physiologically relevant experiment conditions, thus providing improved and physiologically relevant results [Link]. The use of the crescent-shaped spindle provides a common and product independent testing environment one observes in vivo, where products are also evaluated under common and product independent environment. This is in contrast with the current practices of using paddle and basket apparatuses where practically each and every product is analyzed using its own method, a physiologically non-relevant condition.
It is, therefore, essential that for accurate dissolution results and their interpretation one should conduct drug dissolution tests using apparatuses which can simulate physiologically relevant experimental conditions. As the paddle and basket apparatuses provide a non-physiological testing environment, they will provide non-physiologically relevant results, thus the lack of the faith.
Drug dissolution testing for phase I clinical trials/studies
I received a query by email seeking my opinion concerning the topic mentioned above. The email is attached (link), without reference of the sender, to provide a background of my response. My opinion is as follows:
First, what is a phase I clinical study? In general, a phase I clinical study is a study in which a drug is to be evaluated in humans for the first time, following successful animals studies, to establish its safety and tolerability in different dosage strengths. In principle, at this stage, there would not be any data available on human pharmacokinetics (absorption, metabolism, elimination, volume of distribution etc), and this phase of the study is generally used for determining these parameters. If the drug is to be administered as a solid oral product such as a tablet/capsule or suspension, then such a product is to be “developed”. [link for full response]
Defining a dissolution tester and need for a reference product
When one mentions or asks for a dissolution tester, immediately the following apparatuses come to mind: paddle, basket (rotating or reciprocating) and flow-through. The question is why are these apparatuses known as dissolution testers? How do these apparatuses measure and describe the dissolution (characteristics) of a drug product? Consider the question in another way. An analyst has a tablet product, e.g., of acetaminophen, and would like to determine dissolution characteristics of the tablets. Can any one of these testers provide the answer, maybe not? … (Link for full article)
Recent exchange of thoughts
Often I receive comments on my posts/views on the subject. Recently, I received an extended commentary in response to one of my recent articles published in the American Pharmaceutical Review. Based on this commentary we had a good exchange of thoughts and ideas. In the end, the thoughts were summarized by Dr. Dimitri Papadimitriou (Full contact information below) about my views and his understanding of the subject in general. There are healthy differences in opinions, which I have highlighted in red as my responses to the comments.
I think readers of the blog may find this discussion and exchange of thoughts interesting and useful, therefore, with due permission I am posting the response and my views (in red) for the benefit of visitors of the blog (link).
Please, consider submitting your ideas, thoughts and/or queries of mutual benefits as well. I look forward to receiving such contributions.
Contributor’s Info:
Dimitri Papadimitriou Ph.D.
Arevno Consulatants
Shreveport, LA 71115
E-mail: arevno@gmail.com
A discriminating dissolution test
A dissolution test may result in two sets of outcomes, one in which the test may be considered as discriminating and others in which it is not. It is only the link to the in vivo characteristics of the test product (blood profiles or bioavailabilities) that will dictate the discriminating ability of the test. For further details please see the publication (Link).