Old traditions take a long time to die!
There is no doubt that drug dissolution testing is an important and useful subject. However, currently used techniques and practices are generally not science-based and do not provide answers to questions people often ask. For example:
When apparatuses (dissolution tester) are sold by vendors, in particular Paddle and Basket, they do not provide evidence that the testers are, indeed, capable of providing dissolution characteristics of a product (e.g. see link). The capability of a tester as a dissolution tester can only be provided if a vendor provides dissolution characteristics of a reference product or a test product given by the purchaser. Presently, the vendors are only providing apparatuses, which meet the expected physical specifications of a reproducible stirrer or mixer. They are not capable of providing the dissolution characteristics of a product in particular for human use.
A formulator or drug developer would not be able to develop a product using the testers, in particular the Paddle and Basket, because testers do not have defined and set experimental conditions capable of reflecting a product’s behavior in vivo (humans). The formulator requires an acceptable set of experimental conditions to evaluate and develop products which are not available.
A manufacturer cannot use these apparatuses to establish the reproducibility or consistency in lot-to-lot production, as consistency of the testing itself is unknown, or at best extremely high.
The evaluator, in particular for regulatory purposes, requires evidence that indeed the dissolution test employed can differentiate between an acceptable product and an un-acceptable product. These acceptability criteria require that dissolution tests should predict, or link, dissolution behavior to the in vivo results i.e., should have a successful IVIVC. However, on the other hand, it is very well known that the current approaches of testing, in particular using the Paddle and Basket apparatuses, almost never predict the in vivo characteristics of a product. Therefore, an evaluator will always have difficulty in making a decision based on the data provided, using these dissolution apparatuses.
The obvious question is why are we continuing with these practices and making claims of “success” and “usefulness” of the current practices? The answer is that old traditions take a long time to die.
Predicting Blood Drug Concentration-Time (C-T) Profiles Using Convolution Technique – Valproic Acid
Predicting Blood Concentrations-Time (C-t) Profiles from Drug Dissolution Results without Developing an IVIVC – Validation
An article written for the blog
Please click here to open the article. Hope you will find it interesting and useful.
Drawing Conclusions from Dissolution Testing/Results – A Cautionary Note
Drug dissolution studies are often reported in the literature as:
(1) Influence of formulation parameters on dissolution rate … (2) Formulation and evaluation of extended release matrix tablets using factorial design (3) Development of novel … controlled release tablets … and the effect of co-excipients on in vitro drug release rates. (4) Change in the drug release behaviour of tablets …
These are few examples of partial titles selected at random from a quick review of literature. The results and conclusions drawn from these studies are all based on in vitro drug dissolution testing only using the paddle or basket apparatuses. There is no harm in conducting in vitro dissolution testing and drawing conclusions from such studies based on the obtained results. However, it is important to note that the conclusions drawn can, and in fact most likely will, be erroneous and misleading. The reason being, that these conclusions always appear to imply that they are reflective of potential in vivo behaviour of the products. In general, such an assumption may be accurate, but in the case of drug dissolution testing it is not valid, as the commonly used apparatuses are not validated apparatuses. Continue reading
Citing a Post
To cite a post from this blog, you may use the following format which is in the Harvard Referencing Style:
“Saeed A. Qureshi. (2010). Determining blood concentration-time (C-t) profiles from in vitro dissolution results and product evaluation–carbamazepine. Available: http://www.drug-dissolution-testing.com/blog/blood%20levels%20carbamazepine.pdf. Last accessed 25h Jan 2010.”
Developing Dissolution Methods for Pharmacopeial Purposes – Deficiencies
Commonly dissolution tests are to be conducted to assess the potential in vivo drug release characteristics of the products in humans. It is to be noted that it is the only objective of conducting a drug dissolution test. However, there has been limited success in achieving this objective because of the currently suggested (mandatory?) apparatuses lack in providing an appropriate in vivo environment, in particular in terms of stirring and mixing. Therefore, rather than addressing this deficiency, unfortunately, the testing is often presented with two made-up objectives, i.e., without a scientific basis, in particular for the tests described in the pharmacopeias (e.g. USP). (1) It is often described that pharmacopeial tests should only be considered as quality control (QC) tests. (2) Moreover, as these QC tests are often not linked to any “quality” attribute or performance of the products; these pharmacopeial tests are then suggested to be considered as tests for monitoring lot-to-lot consistency of the products. Thus, as these so called “QC” or “consistency check” tests have no link to the product attributes, therefore, they can be developed using any of the experimental conditions to meet some arbitrary criteria.
One such arbitrary criterion is that for an IR product about 80% of the drug should be released within 30 to 45 minutes using a Paddle or Basket apparatus. This in essence is the requirement for a pharmacopeial dissolution test. Continue reading
F2 – Similarity Factor (A Deficiency)
The similarity Factor or F2 is a parameter commonly used to show similarity or equivalence of two dissolution profiles. The F2-value is often calculated using the formula described here (link). The values of F2 range from 0 to 100. Commonly, a value between 50 and 100 is considered to reflect the similarity of two dissolution profiles which implies that the products will have similar in vivo drug release characteristics as well.(please click here for complete post).
Some Thoughts on a Recent US FDA Document “Quality by Design for ANDAs: An Example for Modified Release Dosage Forms”
The US FDA (CDER) released a document on the above mentioned title (Link). This single spaced 161-page long document provides an example of conducting and reporting studies for developing generic drug products as per the QbD (Quality by Design) approach.
It appears that this document may also be considered a “How-to manual on drug dissolution testing”, as a significant portion of the document describes the development and application of the dissolution testing.
It may be argued that if current practices of drug dissolution testing would not have faced so many problems/deficiencies and uncertainties, the procedures and documentations provided would certainly be simpler and shorter. Therefore, indirectly, the document may be considered as a long awaited recognition of the fact that current practices of drug dissolution testing are complicated and complex, and may not be working as well as one should expect.(please click here for complete post).
Can an Appropriate Dissolution Method Transfer Protocol be Developed?
In simple terms, a dissolution method transfer protocol (“protocol”) is a description of a mutual understanding of two parties, developer or current user of an analytical method (“originator”) and the receiver (“recipient”) of the method as to how a dissolution test is to be conducted.
There are numerous situations where such protocols are needed, for example, transferring a method from R&D to the QC section, one plant to another plant, manufacturer to contract organization (CRO) or to sub contractor etc. The easiest and most practical approach for developing the protocol appears to be that both parties work together to develop step by step instructions which are able to be followed by current or future analysts to conduct the test as expected to produce consistent results.
The understanding between the two parties reflects how a test is to be conducted and what kind of output should be expected. The protocol can be: (1) simple/verbal understanding between parties such as preparation of a 0.05M phosphate buffer having pH 5.8 as per USP or; (2) detailed and documented (written) step by step set of instructions for conducting the analytical test, e.g., dissolution. In both cases, the common aspect is that the method should be able to provide output which can be compared, for example; the final pH of the buffer solution, or dissolution results. For the comparison of results between two parties most often two sets of values are used which are the mean and standard deviation (STD) describing the characteristics of the test product. Continue reading
Potentially Incorrect Interpretation of In Vitro Dissolution Characteristics of Products – Glimepiride
Often I have written about the deficiencies (flaws) of the Paddle and Basket apparatuses in obtaining relevant and useful dissolution results. The underlying cause of these deficiencies is a poor stirring and mixing environment within dissolution vessels. However, as a long held tradition, these apparatuses are recommended and used for dissolution testing. As the apparatuses do not provide a relevant in vivo environment, obviously in vitro results would not be relevant to in vivo characteristics of drugs and their products. However, to maintain the status quo, the dissolution results obtained are rationalized as legitimate and useful. Considering practice of rationalization of dissolution results and noticing numerous queries in this regard about a drug glimepiride, I came across a publication (link) which may help in explaining the current dilemma of an analyst in dealing with in vitro drug dissolution testing. (please click here for complete post).
Physiological Considerations for Drug Dissolution Testing
Two Useful Resolutions for New Year – 2012
Critical Importance of Stirring/Mixing (churning) in Dissolution Testing
While surfing the Internet, I found two very interesting and useful video clips showing the digestion process in the human gastrointestinal tract. Click on the links to watch the videos (video1 and video2).
After watching these videos, it is very difficult to imagine that people would like to use the currently suggested dissolution apparatuses, in particular paddle and basket. Appropriate dissolution testing would require a fairly rigorous stirring and mixing process, as can be seen in the videos. Currently used apparatuses do not provide any mixing and stirring, in particular the paddle and basket.
An even more irrational and unscientific aspect is that people tend to emphasize the requirement, that the dissolution testing should be conducted using a disturbance free environment. Such requirements are reflected by the suggestions that apparatuses must be free from all forms of (almost nonexistent) vibrations and their effects. Furthermore, if a product moves, or floats, or is expected to move or float, within a dissolution vessel then often it is recommended that it must be caged in a so called a sinker to stop this floating and moving aspect. In addition, it is often recommended that a dissolution test at present should preferably be conducted using a de-aerated medium. It is not clear how this de-aeration step got incorporated in the dissolution testing when it is not relevant to the physiological environment.
From the videos and the comments above, it is clear that the currently used apparatuses provide an incorrect environment for dissolution testing. Furthermore, the suggested experimental conditions, such as de-aeration, vibration free environment and the use of a sinker or fixing product’s position are irrelevant. The data obtained using these apparatuses and the recommended experimental conditions would be of limited or no use.
Best Wishes – 2012
Two-Tier System for Setting Tolerances – (PVT vs Products)
USP introduces a new lot of 10 mg prednisone tablets for Performance Verification Testing (PVT), with the following Acceptance Values, as supplied with certificate (link).
The current practice/approach of setting tolerances appears to be of questionable merit and may require reconsideration, because: (please click here for complete post).
Where does 20% of the drug go?
Most common dissolution/release tolerances for tablet and capsule products as per USP are 80% of the drug dissolved/released at the suggested time. The question is where does the remaining 20% of the drug go, especially, when the product meets the requirement of Assay and CU of 100%. From a consumer/patient perspective, it is like buying a 1L milk carton, but being assured to receive only 800 mL! Please, note that in many cases, the USP tolerances can be as low as 70%.
If USP tolerances are considered correct then a user of the product is expected to receive less and/or inconsistent amount of drug. It is to be noted that all bioavailability/bioequivalence (BA/BE) studies results are reported based on 100% deliverable drug. As Assay and CU show, on average, 100% content and BA/BE assumes delivery of 100% the drug in humans, it clearly indicates that demonstration or requirement of less than 100% in vitro dissolution/release is an inaccurate tolerance (standard).
The reason for this discrepancy (observance of lower in vitro dissolution/release) is because of the poor stirring and mixing environment within the dissolution vessels using paddle and basket stirrers. The poor stirring environment creates unstirred pockets within the vessels where the drug hides. These pockets depend on the nature of the product, in particular excipients, and can hide as much as 40% of the drug as in the case of the dissolution results of the USP Performance Verification Tablets (see Figure). If this flaw of unstirred pockets is addressed, then one can observe release/dissolution of 100% of the drug. This means that not only will dissolution results match with the Assay and CU results, but SEPARATE monitoring of Assay and CU becomes redundant. This leads to simplification, efficiency and accuracy in overall product evaluation and development (see also related links 1, 2, 3).
Pharmacokinetic (PK) Parameters Values for Estimating Blood Drug Concentration-time (C-t) Profiles
Estimating drug conc.-time (C-t) profiles from drug dissolution results requires the use of a few PK parameters of the drug (link). The values for the parameters can be obtained from the literature, however, these values may vary from study to study or source to source. This makes the comparison of results (profiles) between studies difficult. To minimize such differences and for improved comparison, a list of values of these parameters would be useful.
To fulfill this need a new list under the “Useful Lists” section has been added (link). The values described in the list are those which are considered appropriate at this time. However, the list and values are subject to change if and when more appropriate values are suggested. Please consider contributing to the list by submitting suggestions for new additions and/or revisions to moderator@drug-dissolution-testing.com
Crescent-Shaped Spindles – Now Available
 A New Crescent-shaped Spindle for Drug Dissolution Testing—But Why a New Spindle? (Link).
A New Crescent-shaped Spindle for Drug Dissolution Testing—But Why a New Spindle? (Link).
Crescent-shape spindle: Facts sheet (Link)
Advantages of using the crescent shape spindles for drug dissolution testing (Link)
The following links are for the short video clips demonstrating comparative operations of the paddle and the crescent-shape spindles.
Using a disintegrating tablet: Paddle and Crescent-shape spindle
Using a non-disintegrating tablet: Paddle and Crescent-shape spindle
Note: Dimensions may appear slightly distorted
On-site training/demonstration can be arranged. Post-graduate/doctoral fellows who are interested in using the crescent-shaped spindles for their on-going research projects may request samples.
For further information and purchase inquiries please contact by sending an email to (sales@pharmacomechanics.com) or call at: 1-613-797-9815.
A Simple and Unique Approach for Developing and Evaluating Products
Commonly pharmaceutical products are evaluated and developed based on four “quality” parameters/measurements: (1) Identity, to show that a product contains the expected drug; (2) assay, to show that a product contains expected amount of drug (dose); (3) Content Uniformity (CU), to establish that the dose or drug content in each unit varies within an expected range; (4) Dissolution/release, to show that the drug will be released from the product in an expected manner. All these tests are simple chemical tests based on solvent extractions, i.e. the drug is extracted from the product and measured using any of the quantitative techniques such as spectrophotometeric or chromatographic. For complete article, click here.
Drug Dissolution Testing for the Sake of Testing?
Certainly, current practices of drug dissolution testing appear so. Let me explain …
For any analytical technique, there are two basic requirements which it must meet to be considered as an appropriate technique. (1) The technique must be able to provide relevant results and, (2) the technique must be able to provide reproducible results with acceptable variance. In terms of both requirements, dissolution testing would not meet the criteria of an appropriate analytical technique.
For a technique to provide relevant results, it must clearly be linked to a useful and measurable property of the sample. At present, dissolution testing is not linked to a property of the sample (drug product). Currently, it is quite often described as a tool for monitoring the “quality” of drug products, which is similar in concept to monitoring the “quality” of a person, a vague and undefined objective. Therefore, if the objective is vague and undefined then it is not possible to obtain relevant results. Thus, the outcome becomes testing for the sake of testing.
With regard to reproducibility, again, dissolution testing does not meet appropriate requirements. In response to the negative concerns often expressed in literature about the observed excessive variability of testing, suggestions are often made to tighten specifications and/or other controls, e.g. vessel diameter/curvature, removal of all sources of vibration, de-aeration, training of the analysts, and many more. The question would still remain, what should be the acceptable variations of the test and on what basis? Should the %RSD be 1% or 35% or in between and which value should be considered correct and acceptable? The only way to answer this is through multi-lab performance verification tests, such as USP conducts. Unfortunately, USP does not accept the results from its own studies which show lack of acceptable reproducibility of the test. Other regulatory bodies stopped using such a performance test as it does not SHOW acceptable reproducibility. However, there is continued acceptance of the results from the tests based on the assumption that the technique/test provides an acceptable level of reproducibility. It is not clear how, and on what basis that an acceptable level of reproducibility of the test/technique is assumed? Therefore, how would an analyst determine the reproducibility of the test and be able to differentiate it from the variability of his/her product or results. Thus, until and unless, the variability of the underlying test/technique is not known and established, dissolution testing will remain testing for the sake of testing.
It should, therefore, be kept in mind that if an analyst is expected to use dissolution testing, in particular using the paddle/basket apparatus, it is highly likely that the dissolution results will neither be relevant nor reproducible.
Lack of Objectivity and Relevancy of Current Practices
Drug dissolution test as a quality control test: In simple terms, at present a dissolution test as a QC test means conducting a dissolution test as described in a pharmacopeia, in particular USP. If the test meets the pharmacopeial (Tolerances) requirements then the product may be considered a “Quality” product. It is, however, not clear what “quality” the test refers to or to what product property the test is linked to? Therefore, to overcome this lack of objectivity/relevancy, pharmacopeial tests are described as “consistency” tests. Again, it is not clear the “consistency” of which property or parameter the test is refers to?
Dissolution testing during product development: One of the main uses of dissolution testing is to facilitate product development. This use is based on the principle that the tests should be able to provide potential in vivo drug release behavior information. However, this is a commonly recognized fact that currently used dissolution tests generally do not provide in vivo relevant product characteristics. Thus, current practices of dissolution testing at the product development stage appear to lack relevancy and objectivity.
Practices of methods development: For developing a method one requires a well established and accepted reference (product or parameter). In this case, a reference product should be available with known drug dissolution results which are established independently. As there is no reference product with known dissolution results Continue reading
Use of Dissolution Testing During the Product (Tablet/Capsule) Development Stage
Product development stage: What this really means in simple terminology is the stage where a product (formulation + manufacturing process) is developed to show that it is capable of releasing (dissolution) the drug and providing desired drug levels in humans. The drug release characteristics of the product are usually established based on human studies which are commonly known as bioavailability/bioequivalence (BA/BE) studies. However, one requires a simpler in vitro method to screen test products (especially multiple combinations of formulations) to select some (usually one or two) for BA/BE studies.
Drug dissolution test: This is the in vitro test which is used for this purpose i.e., to evaluate potential release characteristics of different products (or formulations). It is, therefore, very important to note that at this stage a formulator must have access to a dissolution method which is capable of reflecting potential in vivo drug release (dissolution) characteristics in humans. This method must already be developed and validated using other well characterized product(s) for human use. In the literature, it is often described that a specific dissolution method be developed at this stage for the particular test drug/product. However, such a practice is scientifically invalid, as a method can only be developed using a product with well established dissolution characteristics. At the product development stage a dissolution test is applied not developed. This is a very important concept, often over looked and should, therefore, be kept in mind. Continue reading
Setting Clinically Relevant Tolerances for IR Products – A Simple and Rationale Approach
For clinically relevant tolerances, perhaps the most important consideration is that the tolerance should reflect consistent and reproducible delivery of an expected dose (amount of the drug) to the patient. Commonly, dosage or strength of a tablet/capsule reflects the expected amount of drug to be released or delivered. Therefore, for clinical relevance, the amount of drug to be released is fixed, i.e. 100% (at least on average). The only variable which needs to be determined is the time, i.e., how long would it take for the drug to be released. For IR products, this duration of drug release is usually an hour or less. However, in exceptional cases, based on experimental evidence, this time duration may be adjusted as required.
The setting of tolerances, therefore, should be based on the duration of time required for the release of all drug present in the product.
It is to be noted that at present, tolerances are set (e.g. see USP) based on two parameters (values) i.e. amount of drug released at a certain time.
The amount (%age of drug) released is often referred to as the Q-value. Although, the Q-value is set based on the product behavior at the product development stage, it is still chosen arbitrarily rather than based on any scientific/clinical relevance. It is not clear why this Q-value is chosen arbitrarily and set at less than 100%, usually 80% or lower, when it should be 100%. The practice of setting tolerances at 80% or less may not be clinically relevant and require reconsideration.
In short, clinically relevant tolerances should only be based on the time duration, i.e., how long would it take for 100% of the drug to be released from a product.
Assay and Content Uniformity (CU) based on dissolution testing (Poster Presentation)
This week I presented a poster at the AAPS 2011 (Washington, DC) meeting describing a study to assess the Assay and CU from a dissolution test. A copy of the poster is attached (link). I hope that you will find it useful and helpful for your own work.
Simplifying Pharmacopeial Tolerances – An Example Based on USP Tolerances for Diltiazem ER Capsules
USP diltiazem (ER) monograph describes 15 sets of tolerances or by definition “the permitted variation in the results (link)”; four sets for 12-hour and eleven sets for 24-hours products. Figures 1 and 2 present these tolerances in a profile format by using the average values of the ranges described. The first and last tolerances where ranges are often described as NMT (not more than) or NLT (not less than) respectively, averages are calculated using zero and NMT values and NLT values and 110 (highest expected percent dissolved from the CU requirement).
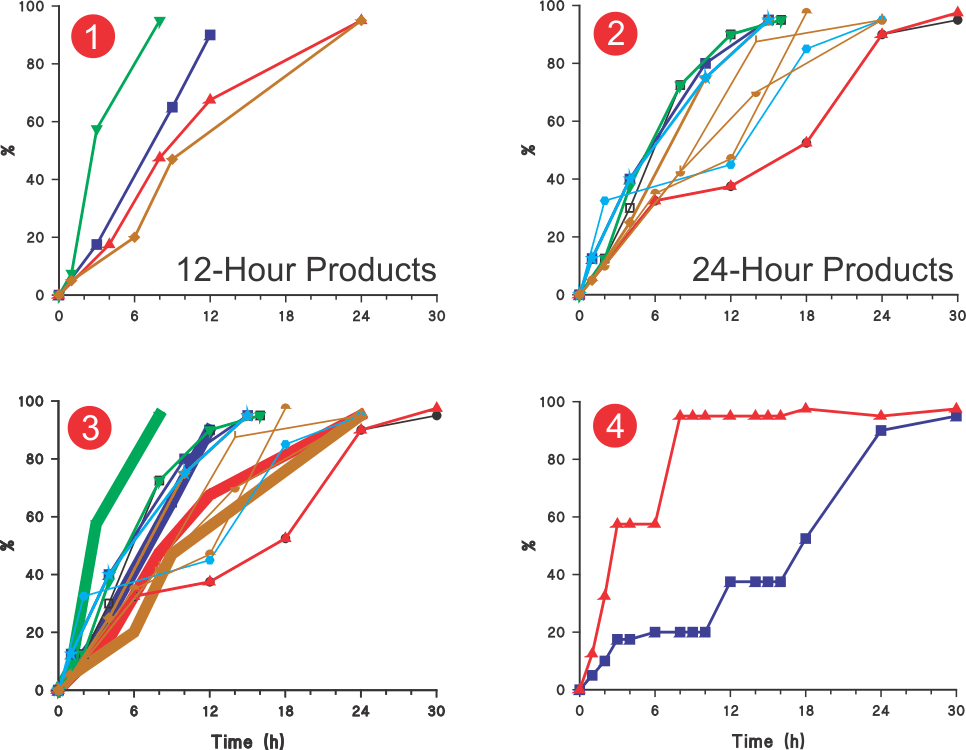
In short, these profiles represent acceptable variation in dissolution characteristics for presumably good quality (without a clinical concern) diltiazem products which are permissible for sale. Although, the monograph describes the tolerances for 12- and 24-h products separately, in most cases these tolerances appear to overlap, as shown in Figure 3. Therefore, it would be safe to assume that, from the pharmacopeial perspective (in vitro testing), both of these types of products will show similar dissolution characteristics!
In most cases, tolerances are based on separate dissolution test conditions. Therefore, it is not possible to ascertain whether the dissolution characteristics are reflective of a product characteristic or because of the experimental conditions used. Therefore, it may not be possible to establish the quality of the product.
On the other hand, if such variations in drug dissolution (release) results are acceptable then the tolerances can be simplified by representing these only with one set of tolerances (upper and lower limit profiles) as shown in Figure 4. This would avoid the current complex and resource intensive practices of setting individual tolerances with no apparent advantage. In addition, it will also help in establishing underlying expected variability in dissolution results.


