Wishing you all –
Objective of drug dissolution testing – keeping it simple and clean
It is very important to note that the objective of dissolution testing is to estimate/determine potential drug dissolution/release characteristics of a drug from a product using a simulated gastrointestinal (GI) tract environment. The simulated GI tract environment for dissolution testing purposes is commonly represented by a medium such as water or an aqueous buffer (e.g. pH=6) in a container (vessel) maintained at 37 °C with gentle mixing/stirring. If the volume of the medium is not sufficient to completely dissolve the expected amount of drug present in the product, then a small amount of solubilizer (e.g. SLS) may be used. Dissolution results are often expressed as percentage of drug released at a single time (e.g. 30 or 45 minutes) or multiple times to establish a dissolution profile.
A dissolution test is just like any other simple analytical test utilizing an instrument, such as a thermometer, viscometer or pH meter that one uses for measuring temperature, viscosity and pH of a product, respectively. One does not require, or develop, a product specific thermometer, viscometer or pH meter. One just puts the thermometer or electrode into the liquid or pours the liquid into the viscometer to obtain the reading. This reading will reflect the property of the liquid.
A dissolution tester should be used exactly like that, i.e. monitoring/measuring of dissolution/release characteristics of a product (tablet/capsule) by dropping a tablet/capsule into a dissolution vessel containing a standard volume of dissolution medium with a stirrer and a pre-set rpm. The outcome of the test (% dissolved) will reflect the dissolution characteristics of the product.
No matter how one presents the argument, or dissolution results, describing them such as bio- or IVIVC relevant, discriminatory, or for QC use, the tests must be performed using pre-set and product independent experimental conditions. This is similar to the practice of not requiring a discriminatory or bio-relevant and/or liquid dependent thermometers, viscometers or pH meters, because not only will it logically and scientifically be considered meaningless but will never reflects true product’s characteristics. Similarly, one should not ask for and/or try to develop product dependent dissolution testers or methods (Please, click here to read in detail about dissolution testing).
Dissolution testing using the crescent shape spindle has been developed based on these thoughts which provide simple, scientifically valid and product independent testing and evaluation. For further information in this regard please see the following links (1, 2).
Promotion of simplicity of paddle/basket apparatuses – A marketing gimmick for scientifically useless and non-validated apparatuses
It is now generally recognised that the commonly recommended dissolution testers (paddle/basket) do not provide bio-relevant dissolution results. In addition, it is also well recognised that considering the flow dynamic within the vessels, these testers will provide highly variable and unpredictable results, thus would be of limited or of no use for routine testing as well.
Considering the need and importance of dissolution testing for product development and assessment, it is surprising that it is often suggested that in the absence of another “simple” alternative, one must keep using these testers. Such a suggestion is simply a marketing gimmick to promote the continued use of a flawed technique. It is almost like bicycle promoters suggesting that until and unless proper boats or ships are developed, people must keep using bicycles to cross rivers, because it is the only simpler and cheaper mean available and allowed for such transportation purposes.
On the other hand, the reality is that it is well known that it is the stirring and mixing mechanism, and environments within dissolution vessels which are causing the problem and require change or adjustment. In this regard, it has been shown that with a simple alteration, for example replacing paddle/basket spindle with the crescent-shaped spindle, not only are the artefacts of the paddle/basket apparatuses corrected but dissolution testing itself becomes relevant and extremely simple. For example: (1) one will be able to conduct product independent tests as opposed to product dependent tests, which is a scientifically incorrect practice to start with; (2) as the testing becomes product independent, one will avoid requiring method developments thus reducing cost and time; (3) the same set of experimental condition will be used for product development and QC purposes, thus again saving cost and time; (4) from the simplicity aspect, testers maintain the simple configuration and operation of the basket/paddle apparatuses.
Therefore, it is important to note that there is no reason that one should continue using a flawed system and keep generating useless data. A simpler and improved dissolution tester can be developed by simply modifying the currently used paddle/basket apparatuses e.g. by replacing a stirring element in it with another, such as with the crescent shape spindle.
Drug Dissolution Testing – A serious concern!
Not very genuine or correct reasons for multiple and product dependent pharmacopeial tests and/or tolerances
While surfing the net, I came across a response to a query, published in Dissolution Technologies, (see issue of February 2011 Volume 18 Issue 1, Question & Answer Section), regarding product specific dissolution testing. It is quite disturbing to read that such poor and irrational scientific reasoning can be provided, for multiple and product dependent, dissolution tests in the pharmacopeia. The suggested reasons are not only scientifically invalid, but also provide a strong case for removing the tests and standards from the compendia which by definition is expected to set un-biased and product independent standards. For example:
It is stated that “We may find tempting the notion that because products may have similar doses and dosing intervals, they should have the same dissolution test. In the present state of the art, that is simply not the case for extended-release products.” On the other hand, such a practice is valid for immediate-release (IR) products, because the same dissolution tests are recommended for IR products (e.g. generics) having similar doses and dosing intervals. It is not clear how a dissolution tester and/or test will differentiate between IR and extended-release (ER) products and will start behaving differently by providing unacceptable results for ER products only. Continue reading
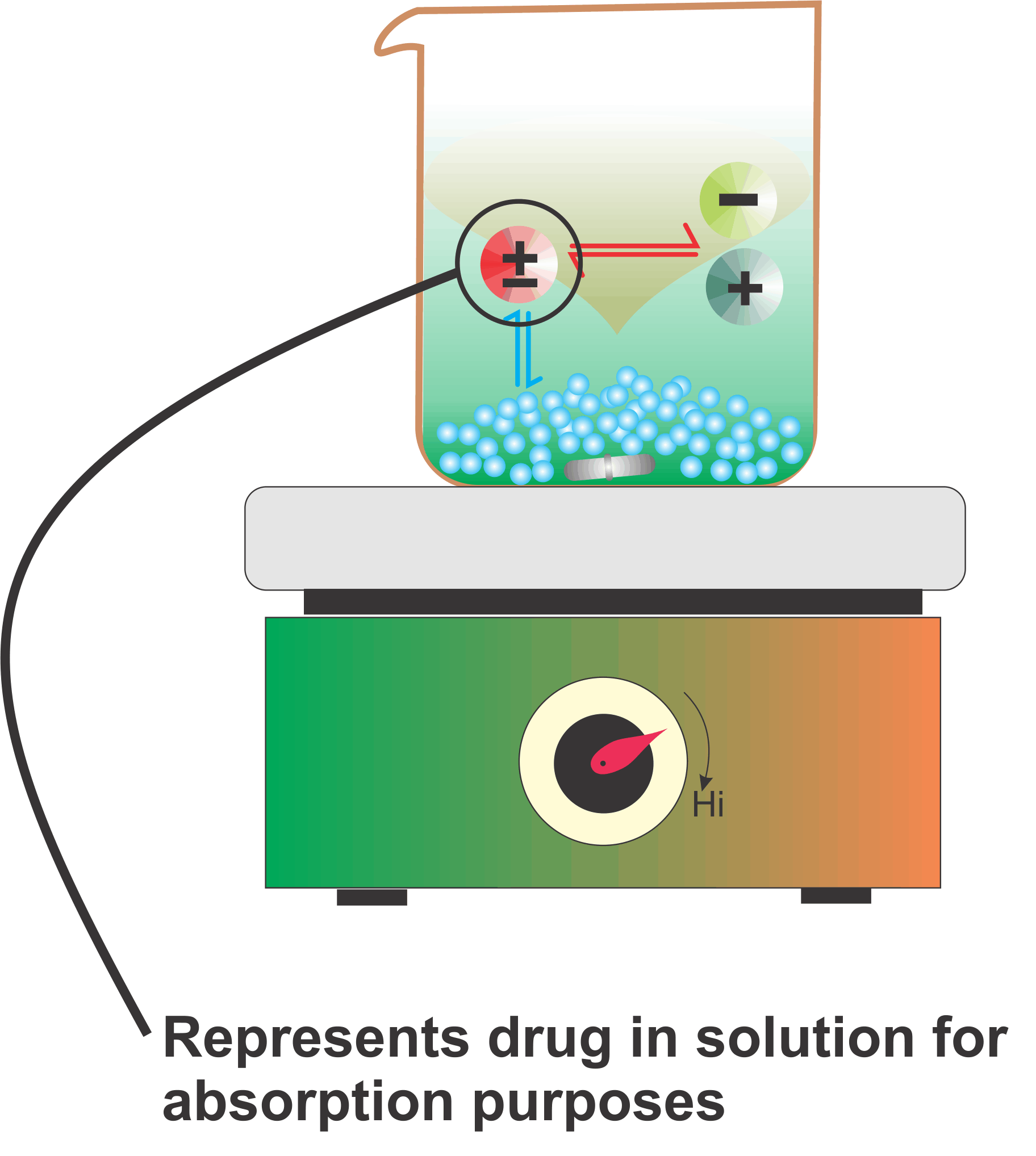
Explaining the solubility aspect of drugs for absorption purposes – another example
The Figure is a simple schematic representation for explaining the solubility of drugs for absorption purposes in the human GI tract for drugs which are weak acids or bases. These drugs dissociate into ions, which are in equilibrium with the undissociated molecules (drugs) in solution.
The undissociated drugs get absorbed which disturbs the equilibrium with the corresponding ions. To maintain the equilibrium the drug moves from the solid to the dissolved (undissociated) form which again gets extracted/absorbed. This cycle continues until the entire drug gets absorbed. The important thing to note here is that for complete absorption, drugs are neither required to be highly soluble nor need a large volume of solvent. It is this continuous extraction/absorption step which makes drug absorption possible and efficient.
For further discussion on the topic please see the following links: (1, 2, 3, 4, 5)
Solubility considerations for drug dissolution testing and product development
Dissolution tests are conducted for solid oral products such as tablets/capsules to simulate/evaluate in vivo drug dissolution which is required for the absorption of drugs from the GI tract to exert their therapeutic effects. Therefore, for appropriate absorption, drugs should dissolve in the liquid present in the GI tract. The liquid present in the GI tract is simulated in vitro with water or aqueous buffers having a pH in the range of 1 to 7.
Commonly in literature three pH values are suggested which are 1, 4.5 and 6.8 to cover the range of pH of the GI tract. It is possible, in fact quite common, that a drug may be freely soluble at one pH but not the other. For example, acidic drugs such as NSAIDs (e.g. ibuprofen) would practically be insoluble in solution having a pH of 1 but will be freely soluble at pH 7. So, how should one decide, for dissolution testing purposes, whether such drugs are of high or low solubility characteristics and how should they be tested? Please click here for complete article
Drug dissolution testing: Limitations of current practices and requirements
To avoid potential frustrations and unnecessary workloads, when conducting dissolution tests one should be watchful of the following limitations of the currently suggested practices and requirements (please follow the links provided within brackets for further details on the topic).
- Mechanical qualification and performance verification testing of apparatuses (paddle/basket) are made up compliance requirements which do not establish that the apparatuses are dissolution testers or capable of providing dissolution characteristics of products (Links: 1, 2, 3).
- Conducting a dissolution test as a QC test, or for checking lot-to-lot consistency of products, is also a made-up requirement or practice and of limited value. Such tests, as conducted presently, are not linked to any of the product quality characteristics (Links: 1, 2, 3).
- Currently used dissolution testers, in particular the paddle and basket, are not qualified and/or validated for dissolution testing purposes (Links: 1, 2).
- Drugs and products specific tests, as currently described in literature, are scientifically invalid (Links: 1, 2).
- Similarly, developing a drug and/or product specific dissolution test is scientifically invalid practice and should be avoided. The practices of developing product specific dissolution methods, and/or using such developed methods, are pretty much waste of time and resources. Following such practices scientists/analysts will never know the dissolution characteristics of a product but determine experimental conditions to achieve desired dissolution results (Links: 1, 2).
- Developing discriminatory tests for detecting formulation and/or manufacturing differences has no meaning or practical use, as products having such differences (e.g. generics) can be bioequivalent and of perfectly acceptable qualities (Links: 1, 2, 3, 4).
- Experimental conditions such as de-aeration, vibration-free environment, use of sinkers etc. can make a dissolution test potentially invalid as these test conditions are not physiologically relevant (Links: 1, 2).
- Developing an in vitro-in vivo correlation (IVIVC) is a futile exercise because dissolution tests are conducted based on the principle that IVIVC always exists (Links: 1, 2, 3).
- Plasma drug levels from dissolution results can only be predicted/estimated using the convolution-based technique. The IVIVC and/or de-convolution techniques cannot be applied/used for such purposes (Links: 1, 2).
- For predicting/estimating plasma drug levels from dissolution results it must be ascertained that results are bio-relevant and obtained using physiologically relevant experimental conditions (Link: 1).
- Biopharmaceutic Classification System (BCS) is based on drug characteristics and not that of the products. Therefore, its use for products evaluation/development may be of limited relevance or use (Link: 1).
- Bio-waivers require that dissolution results must be obtained using bio- or physiologically relevant dissolution tests. As currently described methods are generally not bio- or physiologically relevant, therefore, bio-waivers using such methods should be considered a scientifically weak case (Link: 1).
Absorption mechanism of drugs from the GI tract: Scientific and intuitive considerations
For assessing potential absorption behaviour of drugs from the GI tract, the following points may be useful:
- Drugs are preferentially absorbed as non-ionized (un-dissociated or un-protonated) drug species, therefore, solubility/concentration of the non-ionized species at the site of absorption is to be considered and not that of the ionized (protonated or salt) form. For example, drugs such as diltiazem, metoprolol and propranolol are considered highly water soluble, however, in reality, these are low solubility drugs. The reason for this discrepancy is that these drugs are available and administered as hydrochloride salts, which make them appear highly water soluble. However, following administration in humans, drugs are dissociated from the salt forms depending on the pH of the surrounding environment. They behave according to their native (intrinsic) basic forms, which usually have low aqueous solubilities. In vitro (e.g. for dissolution testing), these drugs may freely dissolve as a salt but in vivo these will behave as native (basic) low solubility drugs. Therefore, in reality in the terminology of BCS, such drugs should belong to the Class II and not Class I, as they are commonly referred to.
- Similarly, an acidic drug such as a propionic acid based NSAID e.g. naproxen as a sodium salt may provide high in vitro aqueous solubility, but would remain a low solubility drug as a native acid just like others such as ibuprofen and diclofenac.
Spindles Videos
The following links are for the short video clips demonstrating comparative operations of the paddle and the crescent-shape spindles.
Using a disintegrating tablet: Paddle and Crescent-shape spindle
Using a non-disintegrating tablet: Paddle and Crescent-shape spindle
Note: Dimensions may appear slightly distorted
Predominance of drugs absorption from the intestinal site compared to the gastric site for both acidic and basic drugs following administration through the oral route.
In an earlier article (link) the mechanism of drug absorption was described considering ionization characteristics of drugs and the differences between the surface areas of the stomach and intestine. The purpose of the article was to explain and highlight how ionization of both an acidic and basic drug will impact in providing undissociated drug molecules both in the stomach and intestine. It is important to note that it is the undissociated drug molecules in the solution form which are responsible, and required, for drug absorption.
The low (acidic) pH of the stomach would favour high undissociated concentrations of acidic drugs compared to high (neutral or basic) pH of the intestine which will favour higher dissociated (ionized) concentrations. The opposite is true for basic drugs where low (acidic) pH of the stomach will result in higher concentrations of ionized or protonated basic drugs in the stomach compared to higher concentrations undissociated drugs in the intestine. The pH of the environments (stomach and intestine) explains only the ionization of drugs (acidic or basic) i.e. comparative availability of undissociated drug molecules in solution form but NOT the EXPECTED absorption of the drugs from these sites. The absorption of drugs, however, can only be explained based on the available surface areas of the stomach and intestine. As the intestine provides a much larger and efficient (permeable) surface, compared to stomach, thus it provides far superior and efficient drug absorption, as explained in the previous article. Continue reading
Impact of dissolution and ionization of drugs, and their interactions, on the absorption through gastrointestinal (GI) tract
This article provides an overview of mechanism of the drug absorption from the GI tract based on solubility/dissolution and dissociation/pH characteristics of a drug. It is argued that although pH values of the environment (stomach and intestine) may play a role, it is the availability of the large surface area of the intestine which predominantly is responsible for the drug absorption for both acidic and basic drugs. Furthermore, in the GI tract drugs exist in three forms i.e. solid (outside solvent/solution) and solid and ions in solution which are in equilibrium with one another. However, it is only the drug in solution form which is relevant for the absorption purpose. The roles of the interactions between drug (solid), drug/ions in solution and the surface areas are discussed in providing efficient drug absorption. Considering the absorption mechanism, the role of in vitro drug dissolution testing is also highlighted.Please click here for complete article
New European (EMA) Draft Guideline
“Guideline on quality of oral modified release products” (link). This is obviously a very important document and a must read. I think it will also help those who require freshening up their understanding of the role and requirements of drug dissolution testing in establishing the “quality” aspect of solid oral drug products.
IVIVC and Predicting of Plasma Drug Levels during Product Development
I have received two or three queries on this topic in recent weeks. I am providing the response with a web-post so that others may benefit from my response as well. The current query is as follows (the name has been deleted and data has been blacked out to keep it confidential):
Qurey:
I read your many excellent articles which guide well the peoples who are new in drug delivery.
After reading a lot of literature on IVIVC, there is still a query in my mind as asked below:
Plasma drug level can be predicted from in vitro dissolution data by two ways:
1. Using convolution approach
2. Using IVIVC
I know how to predict plasma drug level using convolution approach but don’t know how to calculate from IVIVC.
In this context, I need your guidance.
Suppose I have established Level A IVIVC for a tablet formulation with following outcomes; Y = x.xxxX – x.xxx, R2 = 0.xxx.
Then I changed an excipient and did the dissolution testing for new tablet. The new dissolution data is attached. Its outcomes are as; Y = x.xxxX – x.xxx, R2 = 0.xxx.
Now, how can I predict plasma drug level for this new tablets using previously established IVIVC.
Thanking you in advance and regards
Response:
Please, note that plasma drug levels can only be predicted/estimated using the convolution method. IVIVC cannot be used to predict plasma drug levels. I realize that there has been significant promotion to this effect, but unfortunately it is not correct. Furthermore, IVIVC is also of limited, or of no use, during the product development stage, where prediction/estimation of plasma levels is required and the convolution method is the only option for obtaining the required results.
For further information on this topic the following articles may be of help.
http://www.drug-dissolution-testing.com/?p=1648
http://www.drug-dissolution-testing.com/?p=1643
http://www.drug-dissolution-testing.com/?p=833
The killing of drug dissolution testing: what it means and how to achieve this objective.
The following comments are noted from one of my earlier posts, as reported in the FDA transcripts (link):
(1) “It is noted that literally 50 percent of the batches are thrown out every year because of dissolution failures, …”
(2) “There is no evidence that the products out there on the market are bad products. There is no evidence that the agency has done a bad job in serving as a surrogate for ensuring good quality products for the consumer. And, there is no evidence that industry is not focused on quality as an important attribute to manufacturing products.”
Putting these two together clearly shows that we are dealing with the problem of dissolution and not of products or industry? Please click here for complete post
Transcripts of two FDA meetings (held in 2005) on the topic of QbD and Drug Dissolution Testing
People, who are not familiar with the recent history of dissolution testing and QbD may find the following two links useful. These links are for the transcripts of two FDA meetings (held in 2005) on the topic. These are quite long documents and worth reading every word of it. I have noted some of the quotes which may be quite interesting (shocking!). I am of the view that the main or one of the main reasons of starting QbD was to determine and address the issues of drug dissolution testing, in a systematic way based on valid statistical design and analysis (aka QbD). I wonder what happened to that objective and where have we been lost!!!
http://www.fda.gov/ohrms/dockets/ac/05/transcripts/2005-4137T1.pdf
http://www.fda.gov/ohrms/dockets/ac/05/transcripts/2005-4187T1.pdf
Some comments from the speakers:
Dr. Helen Winkle:
“There is no evidence that the products out there on the market are bad products. There is no evidence that the agency has done a bad job in serving as a surrogate for ensuring good quality products for the consumer. And, there is no evidence that industry is not focused on quality as an important attribute to manufacturing products.”
“I think this meeting brings us a step closer to understanding quality-by-design, especially as it relates to dissolution. I think it is really important. I think the whole topic today will really help open the door to us to move ahead in the area of dissolution, and I think we have learned a lot through our past meetings here.”
“The meeting topics that we have for this particular meeting are that we are going to talk about quality-by-design and control of drug dissolution.”
Dr. Moheb Nasr:
“ … that there rate of drug release from solid oral dosage forms is a critical quality attribute.”
“ … that you approve of our approach of implementing quality-by-design in setting dissolution specification.”
Dr. Ajaz Hussain:
“It is noted that literally 50 percent of the batches are thrown out every year because of dissolution failures, …”
“I see our colleagues from Health Canada here who have been criticizing this [dissolution test] for a long time. Thank you for coming, sir.”
QbD (Quality by Design): A systematic approach for evaluating and improving a (production) process or marketing of statistical expertise in disguise?
QbD is often promoted as an approach for improving quality, enhancing efficiencies and reducing cost of the manufacturing of pharmaceutical products such as tablets and products. This article provides a critical assessment of this view. It is argued that the promotion appears to be an attempt to market of the expertise in statistical analyses. This distorted view in fact appears to be causing confusion and hindrance in accepting the QbD approach. A discussion is provided highlighting the underlying issues in this regard. Link for the article Please click here for complete article
Dissolution Apparatuses: Compliant vs Qualified and Validated
It appears that there is serious and unfortunate confusion among the dissolution scientists/analysts which implies that the compliance and qualification/validation of apparatuses are one and the same or interchangeable. This is incorrect. The data obtained using apparatuses such as pharmacopeial paddle/basket, which usually are in compliance but NOT qualified/validated, have limited scientific validity and lack relevance to products’ attributes or qualities, as explained below:
A compliant apparatus means that it meets the required specifications commonly set by standard setting organizations (such as pharmacopeias e.g. USP <711>) for the manufacturing and operation of the apparatuses. On the other hand, a qualified and validated apparatus means that it can be used for its intended purpose to evaluate or assess, reproducibly, the characteristics of the product which in this case is drug dissolution testing. The qualification/validation step usually requires a reference (product) with known characteristics, established independently to the apparatus, which is to be qualified or validated.
It is generally assumed that if an apparatus is in compliance with the required specifications, then it is qualified and validated as well. This is often the case, but not with the dissolution apparatuses. As there is no reference product available with known dissolution characteristics, established independently, one cannot qualify and validate these apparatuses. If one cannot qualify and/or validate a dissolution apparatus, one also cannot determine dissolution characteristics of the test products either. Interpretation of dissolution results obtained from such apparatuses will be misleading at best and incorrect in general. Continue reading
Setting clinically relevant tolerances for dissolution testing: A simple and practical alternative
The in vitro drug dissolution tests, or simply dissolution tests, are conducted to evaluate potential drug release characteristics of a product in vivo or in the GI (gastrointestinal) tract. This in vivo dissolution is indirectly measured based on the observed plasma drug levels or profiles in humans. The drug levels in plasma provide the therapeutic (or toxic) effects thus representing the clinical outcome. Equal or similar drug levels in plasma are considered to provide equal or similar therapeutic effects and vice versa. Therefore, to have clinically relevant dissolution tolerances, dissolution results are to be linked to plasma drug levels. Please click here for complete article
Why is it that QbD in its current form will not help in improving the quality of products (tablets/capsules), and what may be done about it?
This article presents a practical view on QbD (Quality by Design) approach and its implementation. It is argued that, the critical component of the approach, the defined “quality” attribute to be achieved is lacking. To address this issue, from the consumer/patient perspective the quality of a tablet/capsule product may be defined as availability/release of the drug in an expected amount and manner. However, the technique most often used (known as drug dissolution testing) to evaluate such quality has been recognized to be flawed. Therefore, it is highly unlikely that the QbD approach as presented will be successful in providing improved quality of the products. Suggestions are made for addressing the issues for a potentially successful implementation of the QbD practice. Please click here for complete article
Slides from a recent FDA meeting on dissolution Testing
A set of slide presentations from FDA Scientists at the “Advisory Committee for Pharmaceutical Science and Clinical Pharmacology” held on August 8, 2012. (link)
Impressive and highly complicated and complex material. Unfortunately, crescent shaped spindle (http://www.drug-dissolution-testing.com/?p=1136) and simple convolution approach (http://www.drug-dissolution-testing.com/?p=601) did not make it up to there, perhaps it was too simple and straight forward in concept which may actually solve the issues highlighted in the presentations.
Well may be the next time!
Drug dissolution testing and QbD (Quality by Design)
In a recent interaction on a LinkedIn group “Quality-by-Design” under “All it will take is the development of a relevant dissolution method!” I posted a response which I thinkl would be useful to the visitors of this site. Please click here to see the post. Hope you will find it useful.
Costly mistake formulators/analysts often make i.e. developing a product dependent dissolution test
As a fundamental principle of science, this should be quite obvious that one should NOT develop or use product dependent methods or parameters to characterise the product itself. However, this is precisely the practice in the pharmaceutical area for product development and/or its evaluation i.e. everyone seeks/develops and uses a product dependent dissolution method.
This is clearly an example of a mindset which is obviously incorrect and scientifically invalid. It appears that this mindset has been created by the practice of pharmacopeial testing. Most pharmacopeial tests (e.g. USP) are drug and/or product dependent, however, these should be considered scientifically invalid or useless. The reason being that if a dissolution test is product dependent, then, it will not be possible to establish whether observed dissolution characteristics are because of the product or due to the experimental conditions used. Therefore, it should be noted that one cannot rely on product dependent (e.g. pharmacopeial) methods to establish dissolution characteristics of a product, thus its quality.
To evaluate the quality of a drug product, the dissolution method must be product independent. Therefore, developing product dependent dissolution methods for any purpose i.e. QC, discriminatory, bio-relevant, IVIVC, bio-waiver, QbD etc should be considered a mistake and complete waste of time and resources.
At present, vessel based dissolution tester with crescent shape spindles (link) has been suggested for product independent dissolution testing, thus not only it provides unbiased and scientifically valid dissolution testing, but also help in saving large resources.
Presently, only the use of the crescent shape spindle provides true dissolution characteristics of a product
Commonly described dissolution methods are product dependent. Therefore, it is not possible to know whether the observed dissolution characteristics are a reflection of the product (formulation and/or manufacturing) attributes or because of the experimental conditions (apparatus, rpm, medium etc.) employed.
For an appropriate and accurate assessment of dissolution characteristics of a product, the dissolution method must be product independent. The use of crescent shape spindle has been suggested based on this principle, thus provide true dissolution characteristics of a product. Please see the following links, for further discussion:
(1) http://www.drug-dissolution-testing.com/?p=449#more-449
(2) http://www.drug-dissolution-testing.com/blog/files/Flyer.pdf
(3) http://www.drug-dissolution-testing.com/?p=826
(4) http://www.drug-dissolution-testing.com/?p=1136
(5) http://www.drug-dissolution-testing.com/?p=1061#more-1061
Dissolution Testing: Is this the best we got? No, this is the worst which we are required to accept!
A drug dissolution test is an analytical test of such significance that it is hard to imagine that any oral drug product such as tablet and capsule would be developed and manufactured without its use. The majority of the tests are conducted using testers commonly known as paddle and basket apparatuses. It is a well accepted, and implied, understanding that not using one of these apparatuses will require a long and unkind explanation for deviating from the “norms” resulting in potentially extensive and costly delays in bringing the products to the market. Therefore, Please click here for complete article