Monthly Archives: October 2015
Drug Dissolution Testing – Basic Principles & Practices
Attached is a copy of a presentation (slides) given last month at the Rowan University (New Jersey, United States) as a guest speaker for an online course (link).
is a copy of a presentation (slides) given last month at the Rowan University (New Jersey, United States) as a guest speaker for an online course (link).
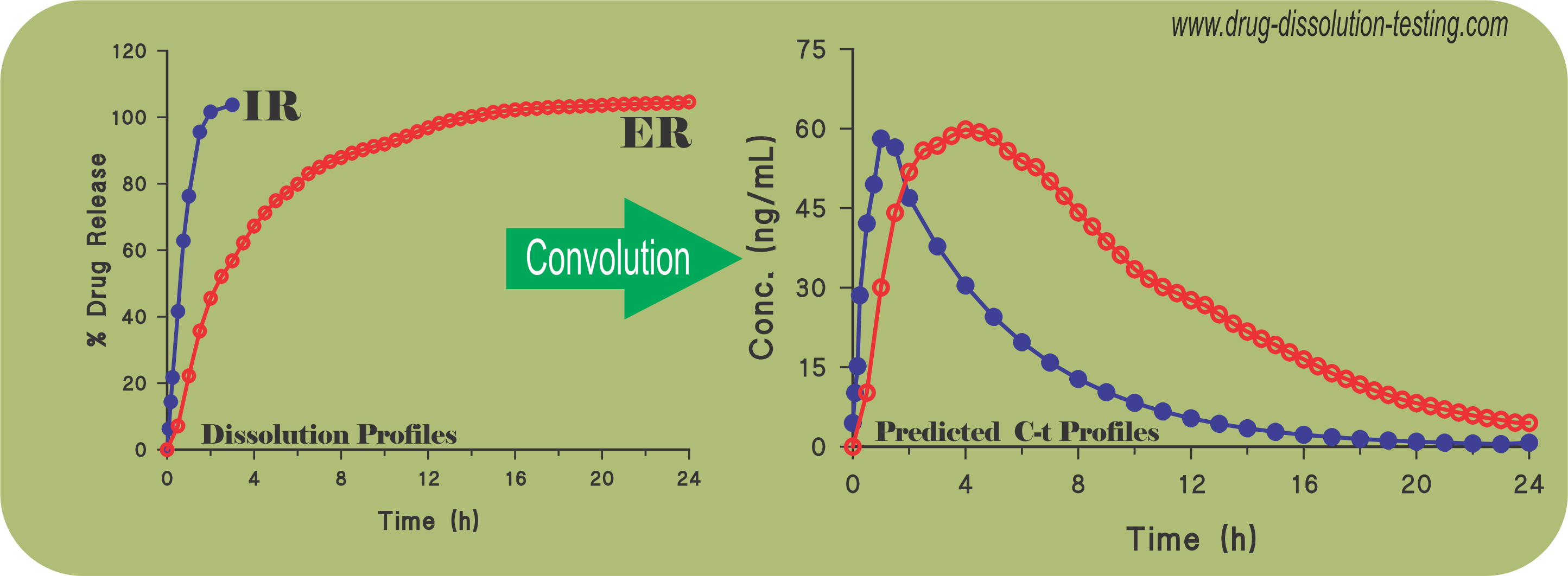
Application (validation) of convolution technique using spreadsheet software for predicting plasma drug levels from dissolution results – some examples
Recently a simple and practical approach has been suggested to predict plasma drug concentration-time profiles from drug dissolution results (link) without conducting a concurrent bio-study. The following citations describe application and usefulness of such an approach as reported in recent literature (refereed journals) from third-party laboratories.
- Solid self-emulsified nanostructures of Lercanidipine hydrochloride: A potential approach to improve the fraction of the dose absorbed. Journal of Drug Delivery Science and Technology, November 2015 (Link)
- Sunitinib-eluting beads for chemoembolization: Methods for in vitro evaluation of drug release (link).
- In vitro dissolution similarity factor (f2) and in vivo bioequivalence criteria, how and when do they match? Using a BCS class II drug as a simulation example(link).
- Establishment of a Bioequivalence-Indicating Dissolution Specification for Candesartan Cilexetil Tablets Using a Convolution Model (link).
- Study the effect of formulation variables on drug release from hydrophilic matrix tablets of milnacipran and prediction of in-vivo plasma profile (link).
- Prediction of in vivo plasma concentration–time profile from in vitro release data of designed formulations of milnacipran using numerical convolution method (link)
- In vitro to in vivo profiling: an easy idea for biowaiver study (link).
- Metoprolol-Eudragit Microcapsules: Pharmacokinetic Study using Convolution Approach (link)
- Dose regimen of para-aminosalicylic acid gastro-resistant formulation (PAS-GR) in multidrug-resistant tuberculosis (link)
- Preparation of acetaminophen capsules containing beads prepared by hot-melt direct blend coating (link)
Some related articles from this blog:
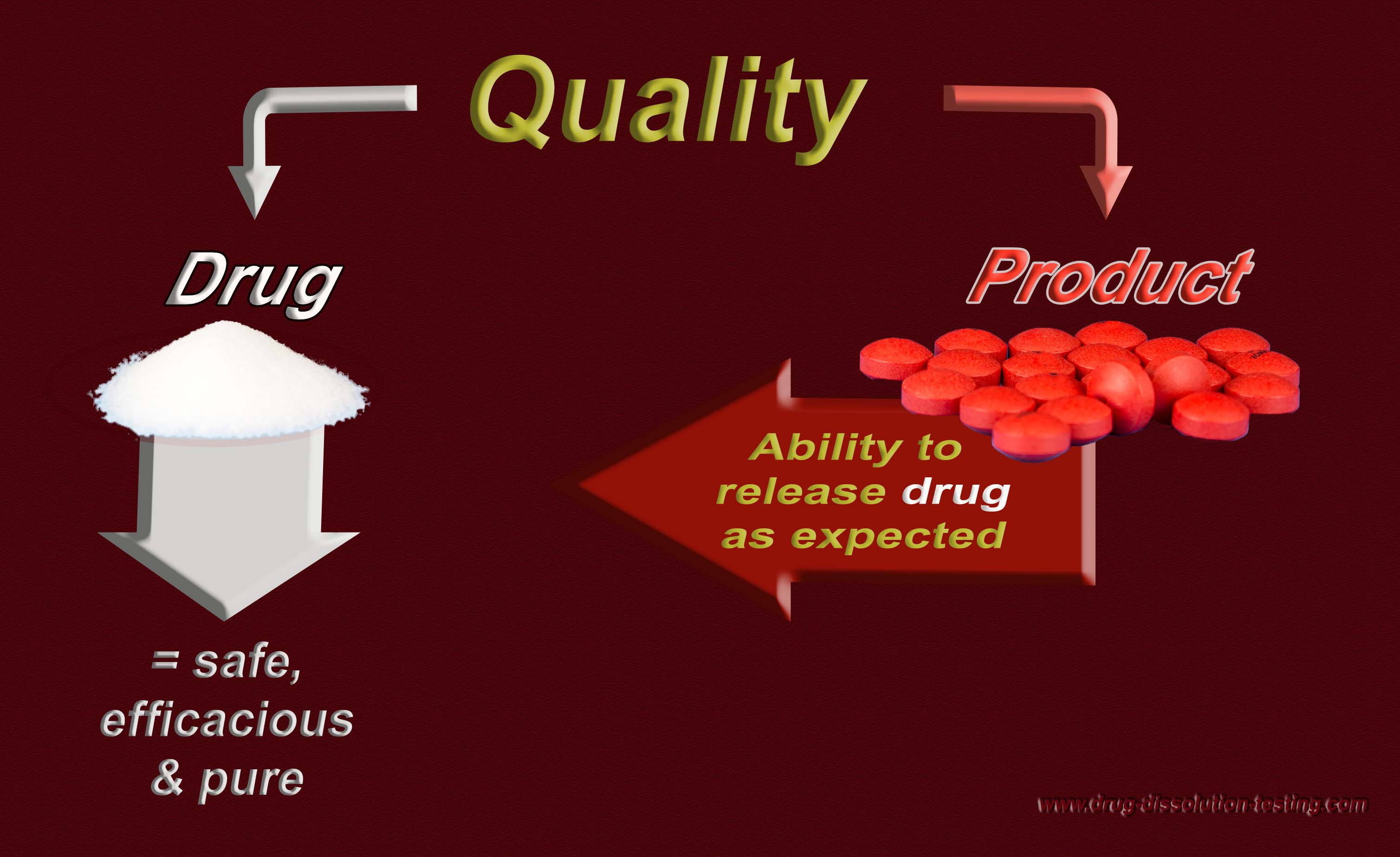
Defining/establishing quality of a drug PRODUCT – the following maybe helpful!
For further discussion, please see the following links (1) http://www.drug-dissolution-testing.com/?p=2359 (2) http://www.drug-dissolution-testing.com/?p=2351 (3) http://www.drug-dissolution-testing.com/?p=2389
Drug Dissolution Testing For Global Bioequivalence Requirements – A Presentation!
Slides from a presentation made at the 2nd Annual Bioequivalence Conference: Intersection between Science and Regulatory Summit, Philadelphia, PA, USA. September 29-30, 2015 link.