Monthly Archives: November 2012
Not very genuine or correct reasons for multiple and product dependent pharmacopeial tests and/or tolerances
While surfing the net, I came across a response to a query, published in Dissolution Technologies, (see issue of February 2011 Volume 18 Issue 1, Question & Answer Section), regarding product specific dissolution testing. It is quite disturbing to read that such poor and irrational scientific reasoning can be provided, for multiple and product dependent, dissolution tests in the pharmacopeia. The suggested reasons are not only scientifically invalid, but also provide a strong case for removing the tests and standards from the compendia which by definition is expected to set un-biased and product independent standards. For example:
It is stated that “We may find tempting the notion that because products may have similar doses and dosing intervals, they should have the same dissolution test. In the present state of the art, that is simply not the case for extended-release products.” On the other hand, such a practice is valid for immediate-release (IR) products, because the same dissolution tests are recommended for IR products (e.g. generics) having similar doses and dosing intervals. It is not clear how a dissolution tester and/or test will differentiate between IR and extended-release (ER) products and will start behaving differently by providing unacceptable results for ER products only. Continue reading
Explaining the solubility aspect of drugs for absorption purposes – another example
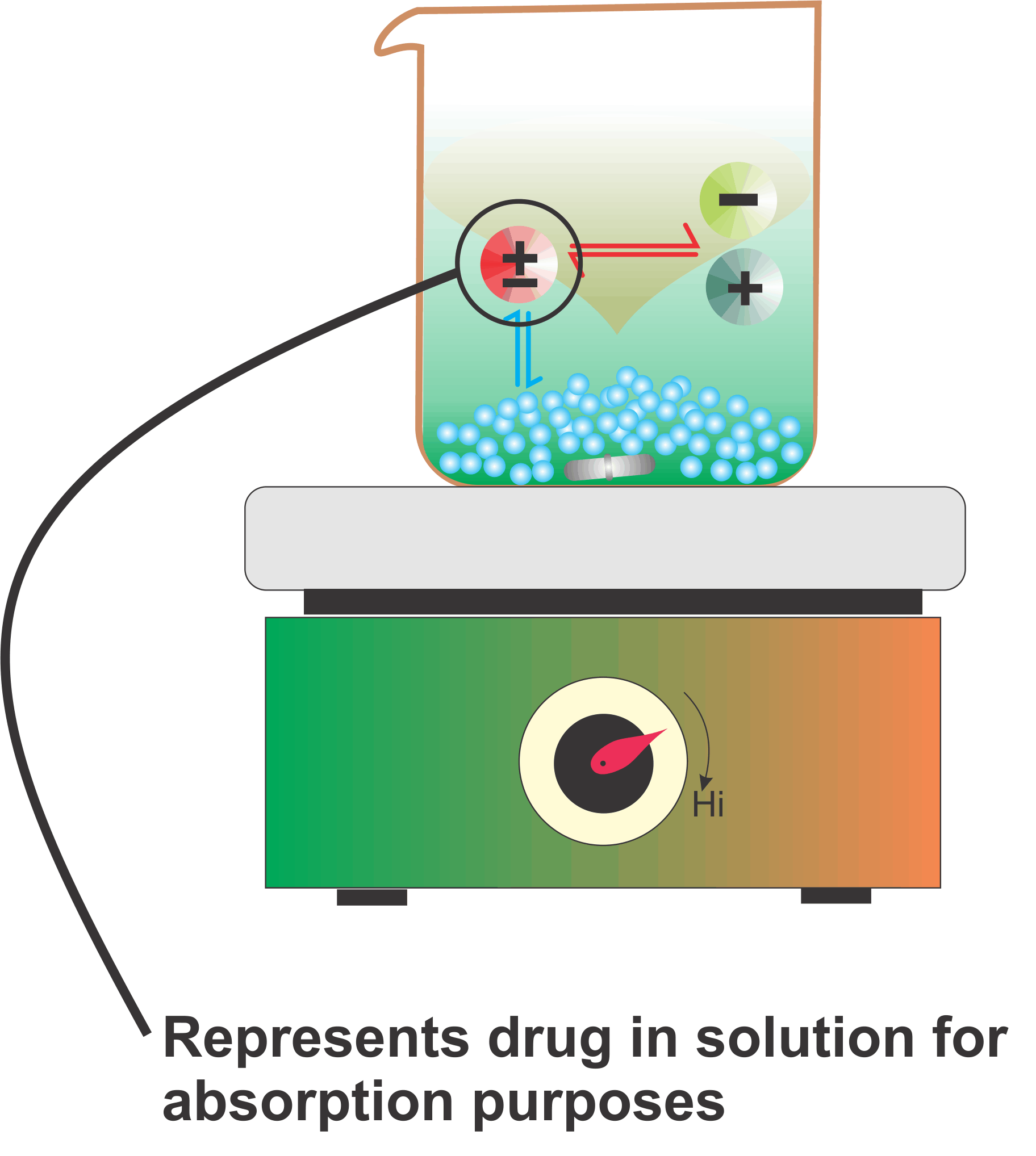
The Figure is a simple schematic representation for explaining the solubility of drugs for absorption purposes in the human GI tract for drugs which are weak acids or bases. These drugs dissociate into ions, which are in equilibrium with the undissociated molecules (drugs) in solution.
The undissociated drugs get absorbed which disturbs the equilibrium with the corresponding ions. To maintain the equilibrium the drug moves from the solid to the dissolved (undissociated) form which again gets extracted/absorbed. This cycle continues until the entire drug gets absorbed. The important thing to note here is that for complete absorption, drugs are neither required to be highly soluble nor need a large volume of solvent. It is this continuous extraction/absorption step which makes drug absorption possible and efficient.
For further discussion on the topic please see the following links: (1, 2, 3, 4, 5)
Solubility considerations for drug dissolution testing and product development
Dissolution tests are conducted for solid oral products such as tablets/capsules to simulate/evaluate in vivo drug dissolution which is required for the absorption of drugs from the GI tract to exert their therapeutic effects. Therefore, for appropriate absorption, drugs should dissolve in the liquid present in the GI tract. The liquid present in the GI tract is simulated in vitro with water or aqueous buffers having a pH in the range of 1 to 7.
Commonly in literature three pH values are suggested which are 1, 4.5 and 6.8 to cover the range of pH of the GI tract. It is possible, in fact quite common, that a drug may be freely soluble at one pH but not the other. For example, acidic drugs such as NSAIDs (e.g. ibuprofen) would practically be insoluble in solution having a pH of 1 but will be freely soluble at pH 7. So, how should one decide, for dissolution testing purposes, whether such drugs are of high or low solubility characteristics and how should they be tested? Please click here for complete article
Drug dissolution testing: Limitations of current practices and requirements
To avoid potential frustrations and unnecessary workloads, when conducting dissolution tests one should be watchful of the following limitations of the currently suggested practices and requirements (please follow the links provided within brackets for further details on the topic).
- Mechanical qualification and performance verification testing of apparatuses (paddle/basket) are made up compliance requirements which do not establish that the apparatuses are dissolution testers or capable of providing dissolution characteristics of products (Links: 1, 2, 3).
- Conducting a dissolution test as a QC test, or for checking lot-to-lot consistency of products, is also a made-up requirement or practice and of limited value. Such tests, as conducted presently, are not linked to any of the product quality characteristics (Links: 1, 2, 3).
- Currently used dissolution testers, in particular the paddle and basket, are not qualified and/or validated for dissolution testing purposes (Links: 1, 2).
- Drugs and products specific tests, as currently described in literature, are scientifically invalid (Links: 1, 2).
- Similarly, developing a drug and/or product specific dissolution test is scientifically invalid practice and should be avoided. The practices of developing product specific dissolution methods, and/or using such developed methods, are pretty much waste of time and resources. Following such practices scientists/analysts will never know the dissolution characteristics of a product but determine experimental conditions to achieve desired dissolution results (Links: 1, 2).
- Developing discriminatory tests for detecting formulation and/or manufacturing differences has no meaning or practical use, as products having such differences (e.g. generics) can be bioequivalent and of perfectly acceptable qualities (Links: 1, 2, 3, 4).
- Experimental conditions such as de-aeration, vibration-free environment, use of sinkers etc. can make a dissolution test potentially invalid as these test conditions are not physiologically relevant (Links: 1, 2).
- Developing an in vitro-in vivo correlation (IVIVC) is a futile exercise because dissolution tests are conducted based on the principle that IVIVC always exists (Links: 1, 2, 3).
- Plasma drug levels from dissolution results can only be predicted/estimated using the convolution-based technique. The IVIVC and/or de-convolution techniques cannot be applied/used for such purposes (Links: 1, 2).
- For predicting/estimating plasma drug levels from dissolution results it must be ascertained that results are bio-relevant and obtained using physiologically relevant experimental conditions (Link: 1).
- Biopharmaceutic Classification System (BCS) is based on drug characteristics and not that of the products. Therefore, its use for products evaluation/development may be of limited relevance or use (Link: 1).
- Bio-waivers require that dissolution results must be obtained using bio- or physiologically relevant dissolution tests. As currently described methods are generally not bio- or physiologically relevant, therefore, bio-waivers using such methods should be considered a scientifically weak case (Link: 1).