Monthly Archives: July 2011
A Concern Which Requires Urgent Attention
Drug dissolution testing is often described as a quality control tool for the assessment of pharmaceutical products, such as tablet and capsule, by establishing batch-to-batch consistency in their production (see). The question is what quality and consistency here one refers to? Saying it in another way, if an analyst conducts a dissolution experiment and obtains certain results, how these results are linked to the quality of the test products. If establishing repeatability/reproducibility/consistency of a product and/or results is the objective, then why can this be achieved ONLY by conducting a dissolution test , when other tests can also be done, e.g., disintegration or grinding (to achieve consistent fine powder). If a product disintegrates or ground to fine powder then expected batch-to-batch consistency is established. There is actually no need to conduct a dissolution test for such a purpose. Manufacturers and regulators have to consider this aspect carefully as to why a dissolution test is necessary to establish batch-to-batch consistency.
On the other hand, if a dissolution test as a solution formation test is needed then perhaps one can do a test using a 50/50 solution of water and ethanol with stirring for half an hour at an rpm of 150 for all products. It is not necessary that the entire drug has to be released or dissolved as long as the results are consistent and reproducible. Such a test can also meet the requirement of a consistency test.
Moreover, a dissolution test is suggested as a consistency test, however, there appears to be nothing consistent about the test itself. For example, one may use any apparatuses (mostly paddle or basket), with any rpm (mostly 50, 75 or 100) using any volume of medium (mostly 250, 500, 900 mL or 1L) having any pH (mostly 1, 4.5, 6.8) of any strength (mostly 0.01 to 0.1M) and having any solubilizing agent (mostly SLS). Further, a dissolution test can be run for any duration of time from 15 minutes to 24 hours. There are no criteria in choosing a consistent experimental condition other than choices based on the discretion of a formulator/analyst to achieve certain DESIRED dissolution characteristics of the product.
In short, a dissolution test as it is conducted or suggested currently does not appear to provide scientific or logical support so that it can be considered as a test to monitor batch-to-batch consistency of pharmaceutical products.
So why is a dissolution test conducted? The only reason the test is to be conducted is to assess in vivo dissolution characteristics of a product. This is a very important concept/requirement which somehow has been overlooked and requires urgent attention.
Considering IVIVC – requires caution
IVIVC is commonly referred to as having an in vitro dissolution method which is able to relate dissolution or absorption properties of a drug in vivo, mostly in humans. It is further assumed that an analyst will be using a paddle or basket apparatus for developing such IVIVC. On the other hand, it is interesting to note that the use of paddle and basket apparatuses has never been validated for establishing IVIVC i.e. whether, or not, these apparatuses are capable of providing relevant in vivo results. Successful examples of such IVIVC are rare, if any. More recent literature highlights that lack of an appropriate stirring/mixing environment within dissolution vessels for these apparatuses may NOT properly reflect in vivo (or bio-relevant) environment thus demonstrating their inability to provide IVIVC.
Therefore, when considering developing IVIVC, or evaluating published literature/studies in this regard, one should be cautious as conclusion drawn could be misleading and/or errounous.
Clarification:
I often receive comments and queries regarding one of my publications on the subject of IVIVC and determining C-t profiles. The method for establishing C-t (blood conc.-time) profiles described in the publication [Link] appears to be quite popular and acceptable. However, it appears that readers are missing a critical aspect of the publication, i.e., the use of crescent-shaped spindle for obtaining the dissolution data which are used for calculating the C-t profiles.
For successful C-t profiles development, it is critical that the in vitro dissolution test method employed must be capable of producing in vivo relevant, or bio-relevant, dissolution results. Unfortunately, as paddle and basket apparatuses are not capable of providing bio-relevant results, the methodology described in my publication may be of limited use or help for the data generated with paddle and basket apparatuses.
Therefore, please make sure that when you determine the C-t profiles, you are using a bio-relevant dissolution tester, and the associated experimental conditions, to generate in vitro dissolution results.
Currently suggested dissolution testers/methods may not be capable of determining dissolution characteristics of drug products.
It is common understanding and practice that an appropriate dissolution test is to be developed for a particular drug product when the product itself is being developed. The rationale being that the product development stage provides sufficient variety of formulation/manufacturing differences along with in vivo (bioavailability/bioequivalence) data to establish the relevance and validity of the proposed dissolution test. The choice of experimental conditions (such as apparatus, rpm, medium, pH, etc) which fit the product’s behaviour the most be chosen as the best or most appropriate dissolution test for future use. If one method may not fulfil the need or requirement, as commonly happens, then two or more methods for the same product may be suggested such that one (simpler) would be used as a QC test and the other (somewhat complex) as bio-relevant. In short, while products are developed, dissolution test(s) are being developed for the product evaluation. Continue reading
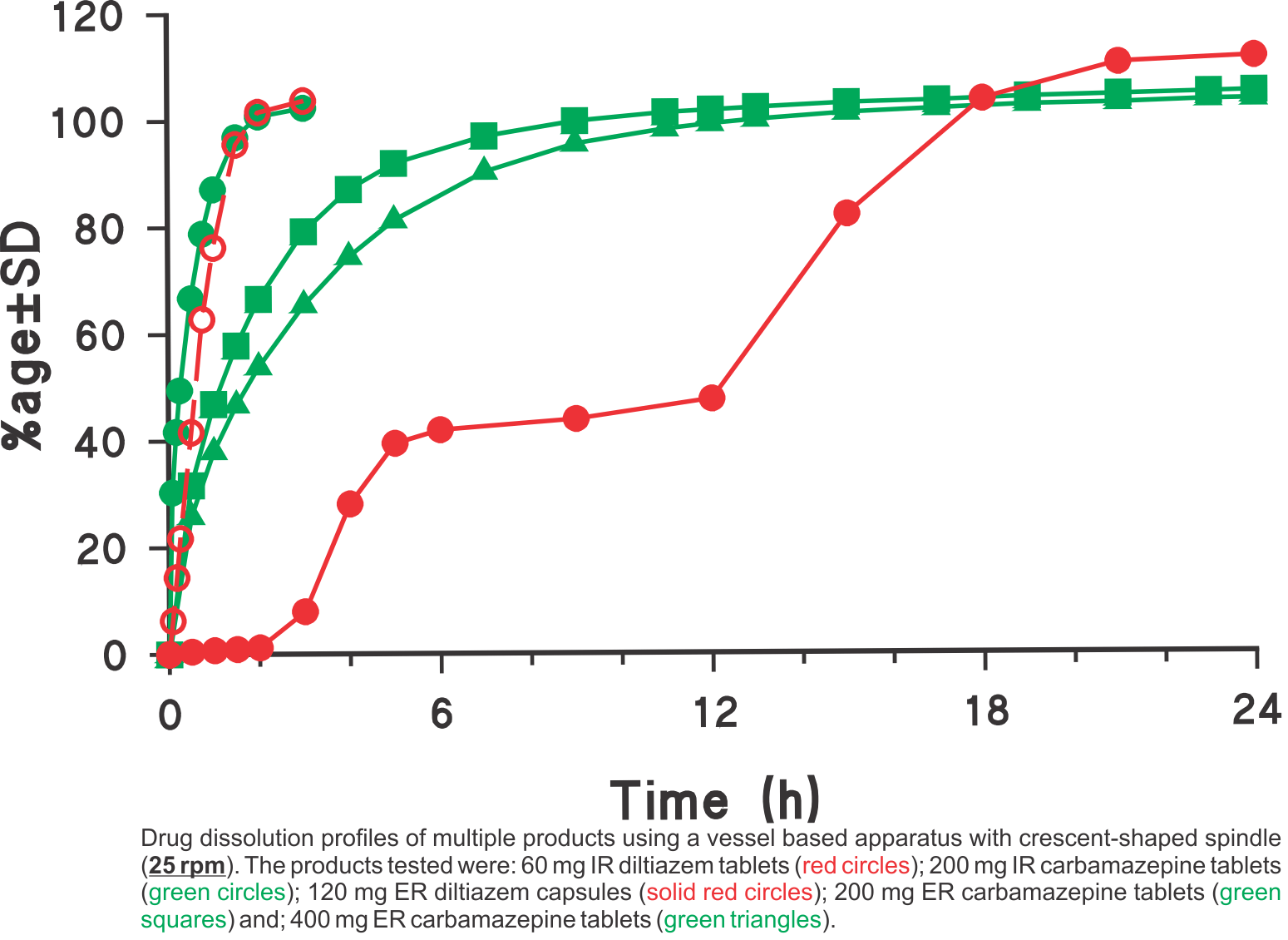
Drug Dissolution Testing Using Simple and Common Experimental Conditions
Dissolution profiles were generated using the USP vessel apparatus with crescent-shaped spindles set at a rotation speed of 25 rpm in all cases. The media used was 900 mL water for diltiazem and 900 mL water containing 0.5% sodium lauryl suphate (SLS) for carbamazepine products, respectively. The SLS was added to provide the needed sink condition. Using the suggested experimental conditions, all one has to do is to provide an appropriate dissolution medium (water with or without a solubilising agent e.g. SLS) so that the expected amount of drug is soluble in the medium.
This single method/approach was employed for analyzing different types of products: tablets, capsules, IR and ER products having high water solubility (diltiazem) and low solubility (carbamazepine). As these experimental conditions are commonly used and simple, the method may easily be transferred to a QC test along with bio-relevant support of the testing environment. For further explanation and discussion on this topic please refer to the publication (link).