Monthly Archives: May 2011
Defining a dissolution tester and need for a reference product
When one mentions or asks for a dissolution tester, immediately the following apparatuses come to mind: paddle, basket (rotating or reciprocating) and flow-through. The question is why are these apparatuses known as dissolution testers? How do these apparatuses measure and describe the dissolution (characteristics) of a drug product? Consider the question in another way. An analyst has a tablet product, e.g., of acetaminophen, and would like to determine dissolution characteristics of the tablets. Can any one of these testers provide the answer, maybe not? … (Link for full article)
Recent exchange of thoughts
Often I receive comments on my posts/views on the subject. Recently, I received an extended commentary in response to one of my recent articles published in the American Pharmaceutical Review. Based on this commentary we had a good exchange of thoughts and ideas. In the end, the thoughts were summarized by Dr. Dimitri Papadimitriou (Full contact information below) about my views and his understanding of the subject in general. There are healthy differences in opinions, which I have highlighted in red as my responses to the comments.
I think readers of the blog may find this discussion and exchange of thoughts interesting and useful, therefore, with due permission I am posting the response and my views (in red) for the benefit of visitors of the blog (link).
Please, consider submitting your ideas, thoughts and/or queries of mutual benefits as well. I look forward to receiving such contributions.
Contributor’s Info:
Dimitri Papadimitriou Ph.D.
Arevno Consulatants
Shreveport, LA 71115
E-mail: arevno@gmail.com
A discriminating dissolution test
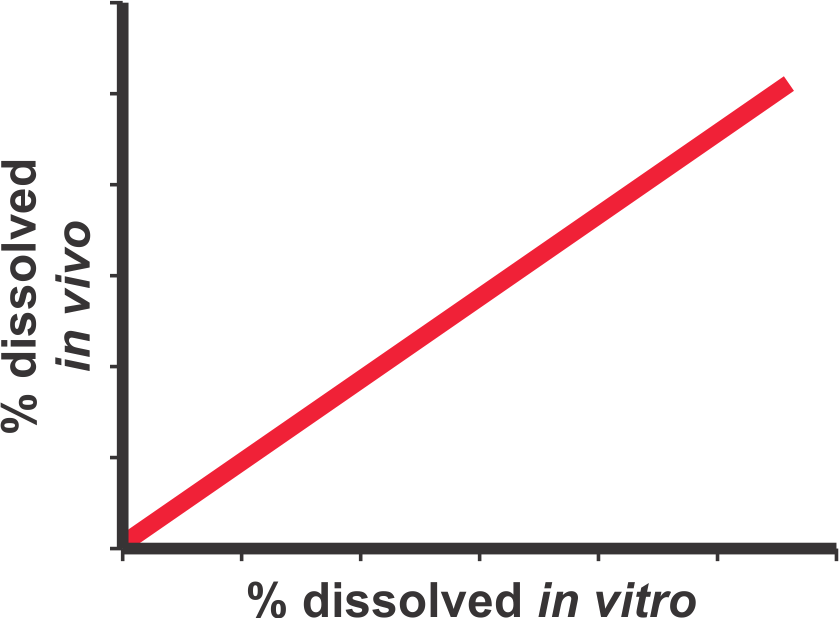
A dissolution test may result in two sets of outcomes, one in which the test may be considered as discriminating and others in which it is not. It is only the link to the in vivo characteristics of the test product (blood profiles or bioavailabilities) that will dictate the discriminating ability of the test. For further details please see the publication (Link).
Are current practices of dissolution testing just to meet the regulatory requirements?
It appears so.
In principle a dissolution test is conducted to assess the quality of a drug product based on its in vitro drug release (dissolution) characteristics. However, in recent years it has clearly been shown, based on experimental as well as computer simulation studies, that the tests, in particular using paddle and basket apparatuses, are not capable of providing relevant and reproducible results. Thus, test results do not reflect the true quality of the products.
Another disturbing aspect in this regard is a concern that most of the tests, i.e., experimental conditions, employed are product dependent. With the use of product dependent experimental conditions it is not possible to establish whether the results, thus, the product quality (good or bad) is a reflection of the product or the use of a particular set of experimental conditions. An analyst, at will, can make a product look good or bad (acceptable/unacceptable) or a product of either slow or fast release type by adjusting the experimental conditions appropriately.
Therefore, current practices of dissolution testing appear to be practices aimed at meeting the regulatory requirements rather than assessing the quality of drug products.
It has further been shown that if the artifacts of the paddle and basket apparatuses are adequately addressed, where apparatuses are able to provide efficient product-medium interaction, a dissolution test can be made a useful analytical tool as intended. For a further detailed discussion on this topic please see the publication.
A bio-relevant dissolution test?
Bio-relevant dissolution tests are highly desirable and are often referred to. It appears that such tests are commonly understood as intuitive expectations rather than a clearly defined scientific objective. This creates confusion, misunderstandings and leads to lack of progress in this regard. To avoid such confusion, the following may be considered as an appropriate definition:
“A bio-relevant dissolution test is a test which is conducted using experimental conditions representing GI tract environment, in particular intestinal, and capable of predicting in vivo response comparable to the actual blood concentration-time profiles obtained from the bioavailability/bioequivalence studies.”
It is essential to understand that a bio-relevant test must be conducted using appropriate and bio-relevant experimental conditions. Use of non-physiological test conditions such as de-aeration of dissolution medium, product dependent experimental conditions, inefficient or lack of stirring/mixing within dissolution vessels, etc., are to be avoided. Furthermore, the test conducted with appropriate test conditions must also be able to predict in vivo (blood) drug concentration-time profiles. For example, the predicted profiles should at least be able to differentiate between fast (e.g. IR) and slow release (ER) products as these would be from an in vivo study. Such a test should then be used to evaluate potential drug release characteristics of a test product. For further discussion of the subject and detailed methodology to calculate drug concentration-time profiles from dissolution results please see the links (1, 2).
Different IVIV Relationship Terminologies
-
IVIVC: (In Vitro-In Vivo Correlation).
This is the most commonly referred terminology and appears to originate from US FDA guidances. It define the IVIVC as, “A predictive mathematical model describing a relationship between an in vitro property of a dosage form (usually the rate or extent of drug dissolution or release) and a relevant in vivo response, e.g., plasma drug concentration or amount of drug absorbed”. Common interpretation: Usually a point-to-point relationship is to be established between the in-vivo and in-vitro parameters of the same time points (e.g., in-vivo fraction drug absorbed vs in-vitro fraction drug dissolved) by applying a mathematical technique of deconvolution. Practical limitations: Both in vitro and in vivo data are required for the same product. The procedure does not provide predictability but reflects relationship between in vitro (dissolution) and in vivo (fraction absorbed/dissolved), if successful. As results are compared/related using a single product, the approach may not be used for the comparison of products, for their formulation/manufacturing attributes. Continue reading
Simplification to a “universal” dissolution test
It is often considered to be highly unlikely, if not impossible, to have a universal dissolution test. Let us explore whether this is a myth or reality?
Most commonly used dissolution methods are those described in the USP monographs (500+) and FDA database (500+). Examined closely, one observes that the majority (~76%) of the tests employ a paddle apparatus at 50 rpm (see US FDA database). Evaluation of USP monographs most likely would also lead to the same conclusion.
As for the dissolution medium, majority (~60%) of the tests employs water or aqueous based buffers in the pH range of 5 to 7.
If one considers the two previous practices together, it appears that we already have a “sort of” universal test which is paddle at 50 rpm using medium having pH in the range 5 to 7, which may be water or a buffer. Furthermore, it should also be noted that these conditions are commonly applied for both IR and ER products. Then why do not we use these “universal” test conditions, and have so many (hundreds) sets of dissolution experimental conditions?
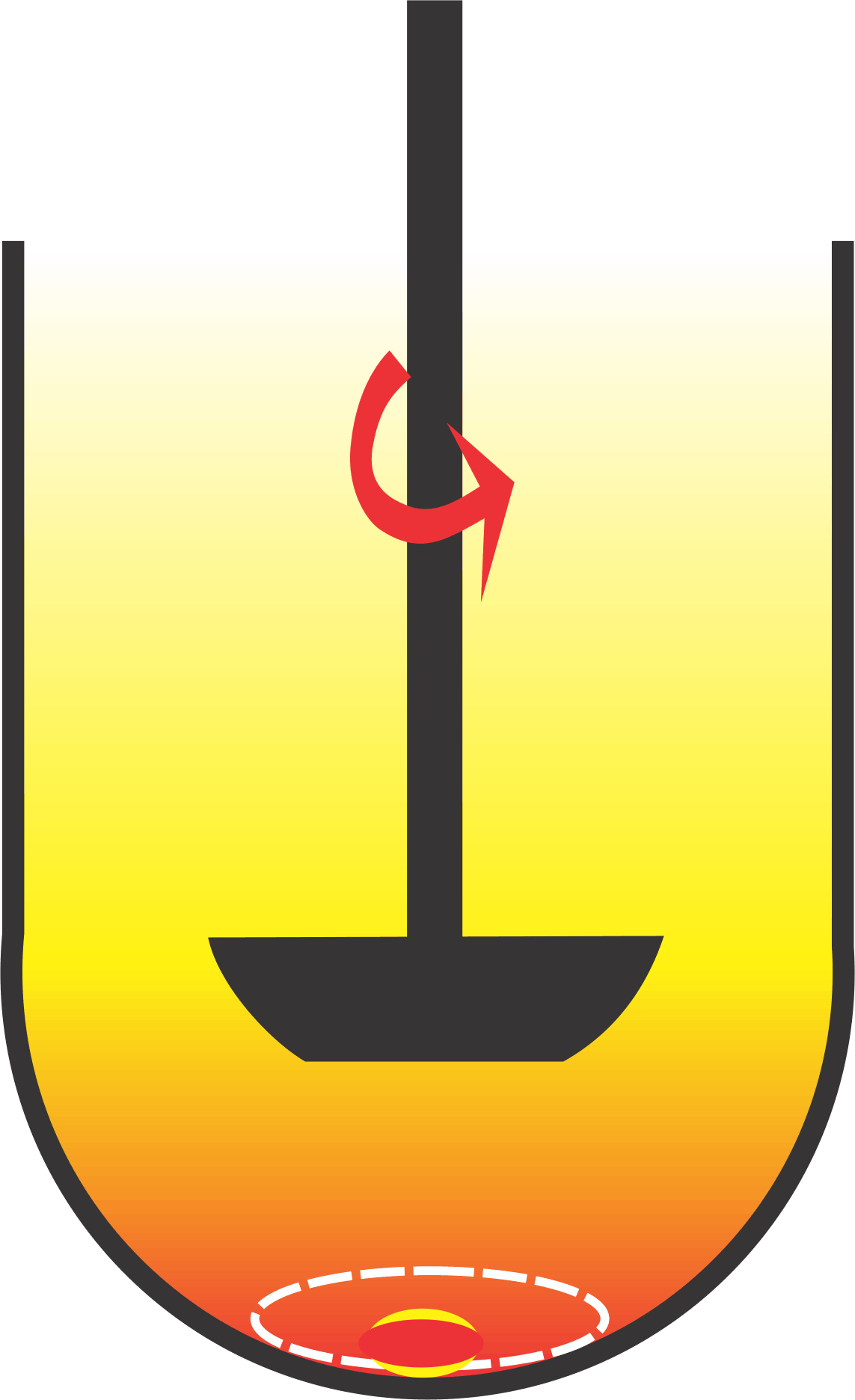
One of the most common deficiencies in using this set of experimental conditions is that the results obtained are often slower and highly variable than expected. This is a normal and expected behavior of paddle spindle as the spindle rotation speed of at 50 rpm does not provide efficient product/medium interaction (figure) and movement of solvent (medium) upwards. The issue in this lies with one of the suggestions to address this limitation, which is to increase the rpm. Therefore a significant number (~18%) of tests are conducted at higher rpm of 75-100. However, there are no established criteria available to select an appropriate rpm, other than to obtain the expected dissolution characteristics of the product. Therefore, an analyst has to select a higher spindle rotation speed (rpm) arbitrarily. Interestingly, this practice of selecting the higher rpm is contrary to the popular belief/recommendation that a desirable characteristic of a dissolution test is that it should be discriminating. Continue reading