Monthly Archives: March 2011
Drug dissolution testing: Mixing by peristaltic motions vs stirring
One of the requirements for dissolution testing is a mixing mechanism to provide efficient product (tablet/capsule) and medium interactions. Within the GI tract such a mixing mechanism is provided by peristaltic compressions and motions.
To evaluate dissolution characteristics in vitro, one needs to provide a mixing mechanism as well. Question is should the in vitro environment have this mixing/stirring based on peristaltic mechanism as well? The answer is not necessarily yes. The reason being that for any in vitro testing, one tries to simulate an in vivo environment or process, but not to duplicate it. This is one of the basic underlying principles of conducting in vitro testing. There are numerous examples of such practices.
For example, in vitro cell growths (cultures) are usually achieved in simple media not in body fluids. Dissolution tests are conducted using simple media (e.g. buffers). Similarly, controlled temperature environments (baths or cabinets) for testing are maintained, including for dissolution testing, using electronic thermostats with heating elements with or without circulating gasses or water. None of these reflect in vivo environments. The mechanisms to simulate the in vivo environments do not require duplication of the physiological (feed-back) processes where such controls are achieved based on enzymatic-based chemical reactions and circulating physiological fluids. It is important to note that arguments (suggestions) of conducting tests by duplicating physiological environments are usually a reflection of lack of appreciation and experience in the physiological aspect of dissolution testing. Continue reading
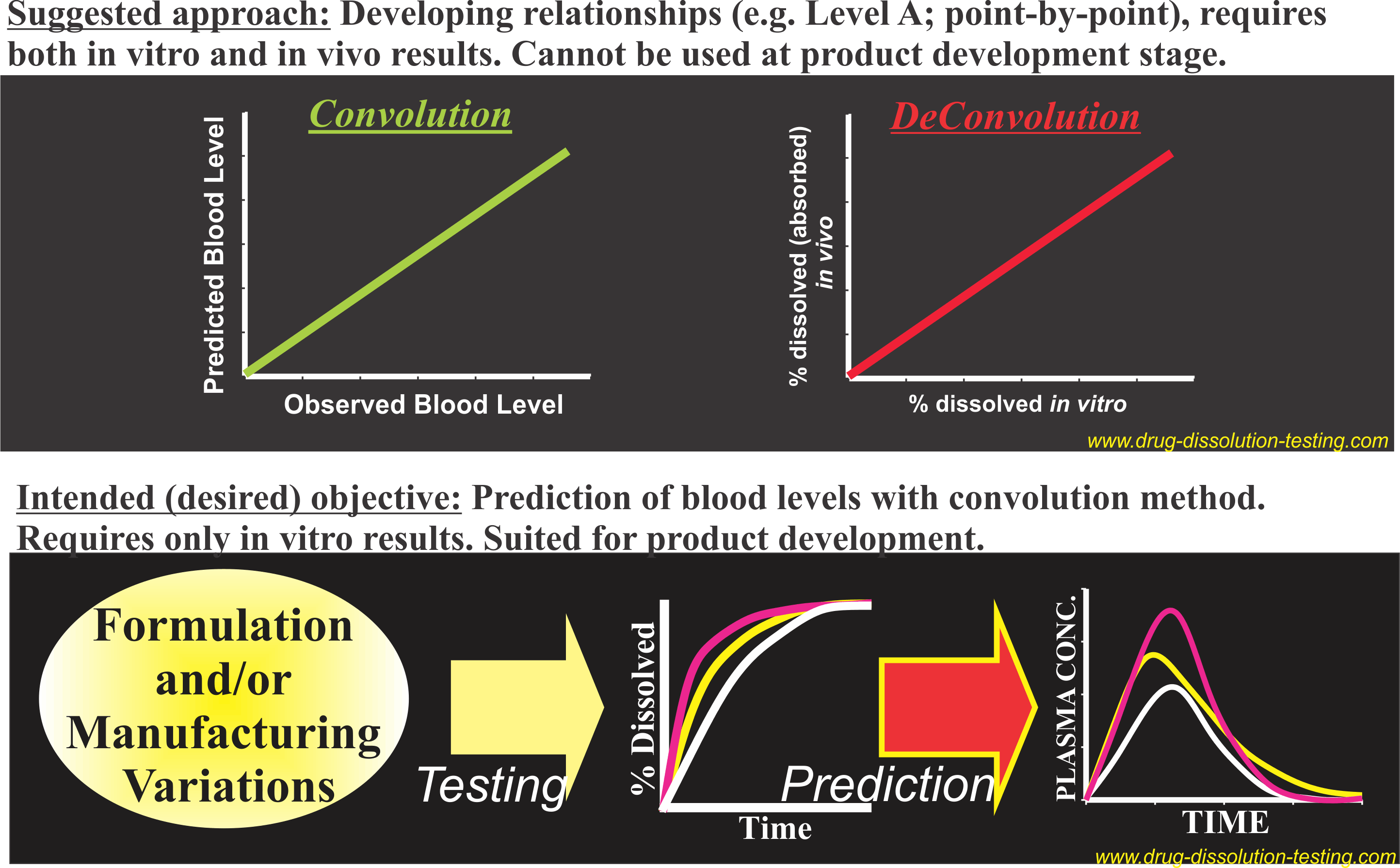
IVIVC – Conflict between practices and objective/intent
For a more detailed discussion on this subject along with a description of a simple method for determining C-t profiles, please see the article (The Open Drug Delivery Journal, 2010, 4, 38-47. (Link).
Product dependent dissolution testing – a scientifically invalid practice
It is often suggested that as drug release mechanisms may differ from product to product dissolution tests conditions/methods, therefore, should also be product dependent to reflect these differences in drug release mechanisms. Often, such reasoning is provided for extended-release products and strangely enough not for immediate-release products. For example, recently (Link, Feb. 2011 issue, Q&A section) such an opinion is provided for nifedipine extended-release tablet products. In reality, however, it is not a correct view and is scientifically invalid as well. Continue reading
Selecting an RPM for dissolution testing
All dissolution apparatuses, or perhaps more accurately dissolution testing in general, have serious problems regarding the lack of appropriate and standardized agitation (stirring and mixing) value. Analysts do not know what should be an appropriate rpm (in the case of Apparatuses 1 and 2), flow rate (in the case of Apparatus 4) and dip-rate (in the case of Apparatus 3). This is a big problem. Every analyst sets this (e.g. rpm) based on his or her preference to achieve some desired dissolution characteristics, thus the test loses its usefulness.
Considering this deficiency, a common agitation speed has been suggested using a new spindle (crescent-shaped). It is to be noted that the crescent-shape spindle was chosen for this purpose because Paddle/Basket would not provide appropriate stirring and mixing. These apparatuses have a design/operation problem, which is very well and extensively documented in the literature. I am of the opinion that if one could make a slight adjustment to the Apparatus 1 and 2 by using crescent-shaped spindle then the current problems in dissolution testing can easily and economically be resolved.
A recent article
Title: Limitations of Some Commonly Described Practices in Drug Dissolution Testing and Suggestions to Address These.
… In conclusion, it may be argued that most of the deficiencies/problems of current practices of dissolution may be related to poor hydrodynamics within the paddle and basket apparatuses which also lack relevance to physiological environment. The dissolution testing may significantly be improved if its role may clearly and objectively be established that the tests are to be conducted only to reflect in vivo dissolution characteristics of a product. This clarity of objective will provide an improved basis for selecting appropriate apparatuses and associated experimental conditions. In addition, such an objective will also reduce significant work load by eliminating requirements of repeated IVIVC developments and other physiologically nonrelevant testing. American Pharmaceutical Review, Jan/Feb. 2011. (link)